
����Ŀ���о�����Ľṹ�Ի�ѧ�²��ϵķ���������Ҫ�ļ�ֵ��
��1����������һ��ԭ�ӵ�����λ�þ����ó�֮Ϊԭ�ӷ�������������ֱ�С��1������x��y��z�������Թ涨��ij����ľ����ṹ��ͼ��ʾ��1��ԭ������Ϊ��0��0��0����2��ԭ������Ϊ��1/3��2/3��0�����þ���Ļ�ѧʽΪ___________���þ�������Ϊ��a = 250.4 pm, c = 666.1 pm���� = 120o�� 3��ԭ������Ϊ_____________���г�����������A��B��ԭ�Ӽ����С�˼��ļ���ʽΪ_____________�������������ֵ������Ҫ����

��2�����������ѻ��ľ����Ǹ������壬����뾶Ϊr��ԭ�ӱ��ֽ��ܽӴ�����������������ݵ��°뾶���Ϊ___________��һ��ԭ�ӡ�
��3��Fe���γɶ������������FeO�����ṹΪNaCl�͡�������ʵ���ϴ��ڿ�λ����λ������ԭ�ӵ�ȱ�ݣ�����ȱ�ݶԾ�������ʻ�����ش�Ӱ�졣���ھ���ȱ�ݣ��ھ�����Fe��O�ĸ����ȷ����˱仯����ΪFexO(x��1)�������ijFexO�����ܶ�Ϊ5.71 g��cm��3�������߳�Ϊ4.28��10��10 m����FexO��x��__________(���������λ��Ч����)��
��4�����ѿ���Ľṹ��ͼ��ʾ������������ӿ���Ӳ��Ӵ�ģ�ͣ������Ӻ���������������ӵĿ�϶���������γ��������壬������λ�������������ģ���һ�������ӱ�__________�������Ӱ�Χ��������λ���������������ģ�һ�������ӱ�_____�������Ӱ�Χ�����ѿ���Ļ�ѧʽΪ__________���������Ӱ뾶Ϊa pm������ѿ��������������Ӽ���̾���Ϊ_______pm��������������Ӽ���̾���Ϊ_______pm��

��5����Ԫ��������ͬ�������壬�������������ѻ��������������������ѻ�����ͼ��ʾF����Ľṹ�У���������a=0.295nm��c=0.469nm�����F������ܶ�Ϊ_____________ g�� cm-3
(��NA��ʾ�����ӵ�������ֵ���г�����ʽ���ɣ����û���)��

���𰸡� AB ��1/3��2/3��1/2�� ![]() ��250.4/3 pm 0.732r��
��250.4/3 pm 0.732r��![]() -1��r 0.92 6 12 CaTiO3 2
-1��r 0.92 6 12 CaTiO3 2![]() a
a ![]() a 2��48/ [(0.295��10-7)2 ��sin60���(0.469��10-7) ��NA]
a 2��48/ [(0.295��10-7)2 ��sin60���(0.469��10-7) ��NA]
��������(1). ���ݾ����ṹͼ��֪����������ռ![]() ����������ռ
����������ռ![]() ����������ռ
����������ռ![]() �������ڲ�����Ϊ�����������У��ݴ˼��㾧��Ļ�ѧʽ��������֪ԭ�����꣬1��ԭ������Ϊ��0��0��0����2��ԭ������Ϊ��1/3��2/3��0����������弸��֪ʶ���3��ԭ�����꣬����ԭ���������A��B��ԭ�Ӽ����С�˼����(2).����������������ı߳������������Խ��ߣ���ϼ���֪ʶ������������������ݵ��°뾶����ԭ�Ӱ뾶��(3). FexO����ľ����ṹΪNaCl�ͣ�����ÿ�������к���4��Oԭ�ӣ�����4����FexO�����ٸ���m=��V������(4).�����������Ӻ���Χ6���������γɰ����壬�����徧������12���⣬ÿ��������1����ԭ�ӣ���12����ԭ�Ӱ�Χ���ĵĸ����ӣ����ݾ�̯�������������и�ԭ����Ŀ��ȷ����ѧʽ���������Ӱ뾶Ϊa pm��������������ⳤΪ2a pm��2�����������������Ϊ�����Σ����������ⳤΪ2a pm��Tiλ�������ε����ģ����������Ӽ���̾�����������ζԽ��ߵij��ȣ�ͼ�����������ĸ������붥����������֮�������̣�Ϊ��Խ��߳��ȵ�һ�룻(5).�����þ�̯������һ�������к��е�ԭ�Ӹ������ٸ�����=
�������ڲ�����Ϊ�����������У��ݴ˼��㾧��Ļ�ѧʽ��������֪ԭ�����꣬1��ԭ������Ϊ��0��0��0����2��ԭ������Ϊ��1/3��2/3��0����������弸��֪ʶ���3��ԭ�����꣬����ԭ���������A��B��ԭ�Ӽ����С�˼����(2).����������������ı߳������������Խ��ߣ���ϼ���֪ʶ������������������ݵ��°뾶����ԭ�Ӱ뾶��(3). FexO����ľ����ṹΪNaCl�ͣ�����ÿ�������к���4��Oԭ�ӣ�����4����FexO�����ٸ���m=��V������(4).�����������Ӻ���Χ6���������γɰ����壬�����徧������12���⣬ÿ��������1����ԭ�ӣ���12����ԭ�Ӱ�Χ���ĵĸ����ӣ����ݾ�̯�������������и�ԭ����Ŀ��ȷ����ѧʽ���������Ӱ뾶Ϊa pm��������������ⳤΪ2a pm��2�����������������Ϊ�����Σ����������ⳤΪ2a pm��Tiλ�������ε����ģ����������Ӽ���̾�����������ζԽ��ߵij��ȣ�ͼ�����������ĸ������붥����������֮�������̣�Ϊ��Խ��߳��ȵ�һ�룻(5).�����þ�̯������һ�������к��е�ԭ�Ӹ������ٸ�����=![]() ������
������
(1). ���ݾ����ṹͼ��֪����������ռ![]() ����������ռ
����������ռ![]() ����������ռ
����������ռ![]() �������ڲ�����Ϊ�����������У���һ�������к���A����ĿΪ4��
�������ڲ�����Ϊ�����������У���һ�������к���A����ĿΪ4��![]() +2��
+2��![]() =2������B����ĿΪ8��
=2������B����ĿΪ8��![]() +1=2����A��B����Ŀ֮��Ϊ1:1�����Ըþ���Ļ�ѧʽΪAB���þ�������Ϊa=250.4pm��c=666.1pm����=120�㣬��֪1��ԭ������Ϊ��0��0��0����2��ԭ������Ϊ��1/3��2/3��0��������ԭ���������д��������ȡֵ��Χ[0��1]֮�䣬�������弸��֪ʶ��3��ԭ����2��ԭ��֮�ϣ����������������м䣬��3��ԭ������Ϊ��1/3��2/3��1/2�������ݾ����ṹ�����½Ƕ���A��������������B���Ӽ�ľ�����̣�����ԭ��������Է��ֵ����Aԭ��λ�������������ģ��������弸��֪ʶ��������Ҷ�����cos120��=
+1=2����A��B����Ŀ֮��Ϊ1:1�����Ըþ���Ļ�ѧʽΪAB���þ�������Ϊa=250.4pm��c=666.1pm����=120�㣬��֪1��ԭ������Ϊ��0��0��0����2��ԭ������Ϊ��1/3��2/3��0��������ԭ���������д��������ȡֵ��Χ[0��1]֮�䣬�������弸��֪ʶ��3��ԭ����2��ԭ��֮�ϣ����������������м䣬��3��ԭ������Ϊ��1/3��2/3��1/2�������ݾ����ṹ�����½Ƕ���A��������������B���Ӽ�ľ�����̣�����ԭ��������Է��ֵ����Aԭ��λ�������������ģ��������弸��֪ʶ��������Ҷ�����cos120��=![]() �����d=
�����d=![]() a�����ǵ�����Խ��ߵľ��룬A��B�ľ���Ϊ��
a�����ǵ�����Խ��ߵľ��룬A��B�ľ���Ϊ��![]() ����A��B֮�����̾���Ϊ
����A��B֮�����̾���Ϊ![]() d=a=
d=a=![]() ��250.4/3 pm���ʴ�Ϊ��AB����1/3��2/3��1/2����
��250.4/3 pm���ʴ�Ϊ��AB����1/3��2/3��1/2����![]() ��250.4/3 pm��
��250.4/3 pm��
(2). ���������֪����������ⳤΪ2r��,������������Խ���Ϊ2![]() r�������������Խ���Ϊ��L2=��2
r�������������Խ���Ϊ��L2=��2![]() r��2+��2r��2��L=2
r��2+��2r��2��L=2![]() r����������������ݵ��°뾶����ԭ��ֱ��Ϊ2
r����������������ݵ��°뾶����ԭ��ֱ��Ϊ2![]() r-2r���뾶Ϊ��2
r-2r���뾶Ϊ��2![]() r-2r��/2=��
r-2r��/2=��![]() -1��r=0.732r���ʴ�0.732r��
-1��r=0.732r���ʴ�0.732r��![]() -1��r��
-1��r��
(3). FexO����ľ����ṹΪNaCl�ͣ�����ÿ�������к���4��Oԭ�ӣ�����4����FexO������m=��V��֪��4��![]() g=5.71 g��cm��3��(4.28��10��8cm)3�����x=0.92���ʴ�Ϊ��0.92��
g=5.71 g��cm��3��(4.28��10��8cm)3�����x=0.92���ʴ�Ϊ��0.92��
(4).������λ�����������Ķ��ǣ���6�������Ӱ�Χ����λ��������������λ���������������ģ���12�������Ӱ�Χ��ÿ�������������Ӻ����Ӿ�Ϊ1����������12�����ϸ���1����ԭ�ӣ����ݾ�̯����֪��ÿ������ռ�е���ԭ����ĿΪ12��![]() =3�����Ļ�ѧʽΪCaTiO3���������Ӱ뾶Ϊa pm��������������ⳤΪ2a pm��2�����������������Ϊ�����Σ��������εı߳�Ϊ2a pm��Tiλ�������ε����ģ����������Ӽ���̾�����������ζԽ��ߵij��ȣ������������Ӽ���̾���Ϊ2
=3�����Ļ�ѧʽΪCaTiO3���������Ӱ뾶Ϊa pm��������������ⳤΪ2a pm��2�����������������Ϊ�����Σ��������εı߳�Ϊ2a pm��Tiλ�������ε����ģ����������Ӽ���̾�����������ζԽ��ߵij��ȣ������������Ӽ���̾���Ϊ2![]() a pm��ͼ�����������ĸ������붥����������֮�������̣�Ϊ��Խ��߳��ȵ�һ�룬�������̾���Ϊ
a pm��ͼ�����������ĸ������붥����������֮�������̣�Ϊ��Խ��߳��ȵ�һ�룬�������̾���Ϊ![]() ��
��![]() ��2
��2![]() a pm=
a pm=![]() a pm���ʴ�Ϊ��6��12��CaTiO3��2
a pm���ʴ�Ϊ��6��12��CaTiO3��2![]() a��
a��![]() a��
a��
(5). ���ݾ����ṹͼ��֪��ÿ�������к���Tiԭ����ĿΪ2��![]() +12��
+12��![]() +3=6��һ�����������Ϊ6��
+3=6��һ�����������Ϊ6��![]() ��(0.295��10��7)2��sin60���(0.496��10��7)cm3=3��(0.295��10��7)2��sin60���(0.496��10��7)cm3�������ܶ�Ϊ
��(0.295��10��7)2��sin60���(0.496��10��7)cm3=3��(0.295��10��7)2��sin60���(0.496��10��7)cm3�������ܶ�Ϊ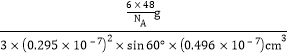 =2��48/ [(0.295��10-7)2 ��sin60���(0.469��10-7) ��NA]���ʴ�Ϊ��2��48/ [(0.295��10-7)2 ��sin60���(0.469��10-7) ��NA]��
=2��48/ [(0.295��10-7)2 ��sin60���(0.469��10-7) ��NA]���ʴ�Ϊ��2��48/ [(0.295��10-7)2 ��sin60���(0.469��10-7) ��NA]��


 ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����SO3��H2O=H2SO4��Ӧ�У���Ӧǰ�����ı����(����)
A.��������B.Ԫ������C.��Ԫ�ػ��ϼ�D.ԭ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Һ����Һ��ȼ�ϵ������������DZͧ����ʾ��ͼ��ͼ��ʾ��
(1)�õ�ص��ܷ�ӦʽΪ_____________���缫1�����ĵ缫��ӦΪ__________��
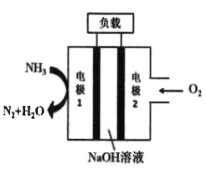
(2)����˵����ȷ����______________��
A. �缫2��������������ԭ��Ӧ
B. ��ع���ʱ��Na����缫1�ƶ�
C. �����ɵ缫2�����·����缫1
D. ��װ�ý���ѧ��ת��Ϊ������ת��Ϊ��е��
(3)����·��ͨ���ĵ�����Ϊ0.4molʱ�������������ı�״�������������Ϊ________L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ѧ�г���X�����о�����ṹ����һ����ɫ����ɱ�ʾΪ��MxFey(CN)z���о��������Ľṹ������Fe2+��Fe3+�ֱ�ռ��������Ķ��㣬�����������ڣ���CNһλ������������ϣ��侧���е������ӽṹ��ͼʾ������˵����ȷ����( )
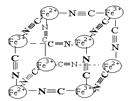
A. �þ�����ԭ�Ӿ���
B. M������λ����������������ģ���+2��
C. M������λ����������������ģ���+1�ۣ���M+��ȱ�ʣ�������û��M+��ռ�����ĵİٷֱȣ�Ϊ50%
D. ����Ļ�ѧʽ�ɱ�ʾΪMFe2(CN)3����MΪ+1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±�ΪԪ�����ڱ���ǰ�����ڵIJ���Ԫ�أ��������е���ĸ�ֱ����һ�ֻ�ѧԪ�أ�����Ҫ��ش����и�С�⣺
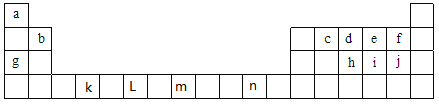
��1����Ԫ�طǽ�����ǿ���Ƚ��кܶ��������f��j�ķǽ�����ǿ�����о������в����е���_________������ţ�
a.�Ƚ����ֵ��ʵ���ɫ b.�Ƚ��⻯����ȶ��� c.������Ԫ�������ڱ���λ��
d.�Ƚϵ縺�� e.�Ƚ�����������Ӧˮ���������
�ڸ���Ԫ��ԭ�ӵ���Χ�����Ų����������ɽ�Ԫ�����ڱ�ǰ������Ԫ�طֳ�4�����ֱ�Ϊs����p����d����ds����������s����Ԫ����_______�֣�����d����Ԫ����_______�֣�Ԫ��n����________����
����c��d��e����Ԫ���У��縺����С�����˳����______________,��һ�������ɴ�С��˳����____________(��Ԫ�ط��Żش�)��
��2����д�� n2���ĺ�������Ų�ʽ��______________________��
��д��kԪ�ػ�̬ԭ�ӵļ۵����Ų�ʽ��_____________��
��д�� LԪ�ػ�̬ԭ�ӵ���Χ�����Ų�ʽ��_____________��
��д��mԪ�ػ�̬ԭ���۵����Ĺ����ʾʽ��________________________����Ԫ����Ԫ�����ڱ��е�λ��Ϊ��__________________________��
��j�������ӵĽṹʾ��ͼΪ____________��
��3����Ԫ��i���⻯���������____________������ԡ��Ǽ��ԡ������ӣ�����ӵĿռ乹��Ϊ____________�����⻯�������iԭ�ӹ�����ӻ�������__________�� ��i��e�γɵ�ie42�����ӣ���ռ乹��Ϊ__________(����������)��
����֪cd- �� d2 �ṹ���ƣ�1 mol cd- ��![]() ����ĿΪ___________����d�γɵ�����d3����CO2��Ϊ�ȵ����壬��d3���ķ��ӹ���Ϊ___________��
����ĿΪ___________����d�γɵ�����d3����CO2��Ϊ�ȵ����壬��d3���ķ��ӹ���Ϊ___________��
��f2ͨ��ϡNaOH��Һ�п�����Of2,Of2���ӹ���Ϊ___________��������ԭ�ӵ��ӻ���ʽΪ_______��
�ܻ�����j2e�����幹��Ϊ_________������ԭ�ӵļ۲���Ӷ���Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���ӷ�Ӧ�漰H2O��Cr2O72-��NO2-��H+��NO3-��Cr3+����������֪��Ӧ������NO3-Ũ�ȱ仯��ͼ��ʾ�����������ͻ�ԭ�������ʵ���֮��Ϊ1��3�������жϴ�����ǣ� ��
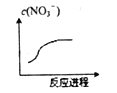
A. Cr2O72-��Cr���ϼ���+6 �� B. ��Ӧ������������NO3-
C. ����1mol��������ת�Ƶ���6mol D. ��Ӧ��Cr3+������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��EΪԭ���������������5�ֶ���������Ԫ�أ����н�����һ�ֽ���Ԫ�أ�A��������Ȼ�����ܶ���С�����壬A��D������������ͬ��BԪ��ԭ�ӽṹʾ��ͼΪ![]() ;C��E�����ڱ������ڣ���E����������C��2������ش��������⣺
;C��E�����ڱ������ڣ���E����������C��2������ش��������⣺
(1)Bλ�ڵ�____________����_____________�壻
(2)E�����ӽṹʾ��ͼ��_________________��DA�к���______________��(�ѧ������)
(3)�õ���ʽ��ʾBC2���γɹ���________________________________________��(�þ���Ԫ�ر�ʾ)
(4)C��D��E����Ԫ�ؼ����ӵ����Ӱ뾶�ɴ�С��˳����______________________��(�þ������ӷ��ű�ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T������.������������������ȡ�
![]()
��ش��������⣺
(1)W�����ڱ��е�λ����____________________________________��Q��R��T����Ԫ��ԭ�Ӱ뾶�ɴ�С��˳��Ϊ____________________________________________________________________��(��Ԫ�ط��ű�ʾ)��QO2�ĵ���ʽΪ________________________��R�����������Ļ�ѧʽ____________________________��
(2)T������NaOH ��Һ��Ӧ�����ӷ���ʽΪ____________________________________________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�����������������ˮ���ǣ� ��
A.MgSO4B.AgClC.Al (OH)3D.BaCO3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com