
����˵����ȷ�� �� ��
�����л�̬ԭ�Ӻ�������Ų�����ѭ����ԭ��
��ͬһ���ڴ����ң�Ԫ�ص�һ�����ܡ��縺�Զ���Խ��Խ��
�����л�ѧ���������������ͬ�����������Ժͱ�����
����������ﶼ������λ�������к���λ���Ļ����ﶼ�������
�����к����Լ��ķ��Ӷ��Ǽ��Է���
���������Ӿ����ж��������Ӽ�
�����н��������۵�϶����ڷ��Ӿ���
A���٢� B���� C���ۢܢ� D���٢ڢ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
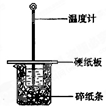 ijʵ��С��ѧ�����տα�ʵ��Ҫ����50mL0.5mol/L������50mL0.5mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų������������к��ȣ�����˵����ȷ�ǣ�������
ijʵ��С��ѧ�����տα�ʵ��Ҫ����50mL0.5mol/L������50mL0.5mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų������������к��ȣ�����˵����ȷ�ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �о�������ұ������������Ҫ���壮
�о�������ұ������������Ҫ���壮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��98%��Ũ�����õ������ˮϡ�ͺ��������������Ϊ49% | B��SO32-��ClO-��NO3-������������Һ������������ԭ��Ӧ�����ܴ������� | C��Ũ���ᡢ�������ǿ�����ԣ�Ũ�����Ũ�����ֽ���в��ȶ��� | D������£�22.4L��������������������Һ��Ӧ��ת�Ƶĵ�����Ϊ2NA����NAΪ�����ӵ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A����״���£���һ���������ĸ�����ƿ����Ȫʵ�飬��ˮ����������ƿ������Һ�����磩����ƿ�ڰ�ˮ�����ʵ���Ũ��Ϊ
| ||
| B����״���£���64gͭƬͶ�뵽��2mol H2SO4��Ũ�����м��Ȼ����22.4L��SO2���� | ||
| C����1mol��̼��ع����л����μӺ�1mol���ʵ�ϡ��������1mol��CO2���� | ||
| D������1mol Ba��OH��2��Һ��ͨ������CO2�������1mol BaCO3��ɫ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com