
вбжЊЯТСаШШЛЏбЇЗНГЬЪНЃК
(1)CH3COOH(l)ЃЋ2O2(g)==2CO2(g)ЃЋ2H2O(l)ЁЁІЄH1ЃНЃ870.3 kJЁЄmolЃ1
(2)C(s)ЃЋO2(g)===CO2(g) ІЄH2ЃНЃ393.5 kJЁЄmolЃ1
(3)H2(g)ЃЋ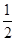 O2(g)===H2O(l)
ІЄH3ЃНЃ285.8 kJЁЄmolЃ1ЃЌ
O2(g)===H2O(l)
ІЄH3ЃНЃ285.8 kJЁЄmolЃ1ЃЌ
дђЗДгІ2C(s)ЃЋ2H2(g)ЃЋO2(g)===CH3COOH(l)ЕФьЪБфІЄHЮЊ
AЃЎ+ 488.3 kJЁЄmolЃ1ЁЁЁЁЁЁЁЁЁЁЁЁ BЃЎЃ244.15 kJЁЄmolЃ1
CЃЎ+ 244.15 kJЁЄmolЃ1 DЃЎЃ488.3 kJЁЄmolЃ1
 ЦпаЧЭМЪщПкЫуЫйЫуЬьЬьСЗЯЕСаД№АИ
ЦпаЧЭМЪщПкЫуЫйЫуЬьЬьСЗЯЕСаД№АИ ГѕжабЇвЕПМЪдЕМгыСЗЯЕСаД№АИ
ГѕжабЇвЕПМЪдЕМгыСЗЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| 1 |
| 2 |
| 1 |
| 2 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| 1 |
| 2 |
| 1 |
| 2 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
 УКЬППЩвдзЊЛЏЮЊЧхНрФмдДКЭЛЏЙЄдСЯЃЎ
УКЬППЩвдзЊЛЏЮЊЧхНрФмдДКЭЛЏЙЄдСЯЃЎ| 1 | 2 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com