
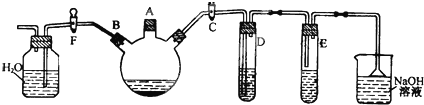
£Ø10·Ö£©Ä³æĪĶāŃŠ¾æŠŌѧĻ°Š”×éÓĆĻĀĶ¼ĖłŹ¾×°ÖĆÖʱøÉŁĮæäå±½²¢ŃéÖ¤äåÓė±½µÄ·“Ó¦ŹĒČ”“ś·“Ó¦”£
ŹµŃ鏱£¬¹Ų±ÕF»īČū£¬“ņæŖC»īČū£¬ŌŚ×°ÓŠÉŁĮæ±½µÄČżæŚÉÕĘæÖŠÓÉAæŚ¼ÓČėÉŁĮæŅŗä壬ŌŁ¼ÓČėÉŁĮæĢśŠ¼£¬Čū×”AæŚ”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£© DŹŌ¹ÜČװµÄŹĒ £¬Ęä×÷ÓĆŹĒ ”£
£Ø2£© EŹŌ¹ÜČװµÄŹĒ ”£
£Ø3£© ³żČ„äå±½ÖŠ»ģÓŠµÄBr2ŌÓÖŹµÄŹŌ¼ĮŹĒ £¬²Ł×÷·½·ØĪŖ ”£
£Ø4£© ČżæŚÉÕĘæÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
£Ø5£© øł¾ŻŹ²Ć“ĻÖĻóæÉÖ¤Ć÷ŃéÖ¤äåÓė±½µÄ·“Ó¦ŹĒČ”“ś·“Ó¦£æ ”£
 ĘŚÄ©ŗĆ³É¼ØĻµĮŠ“š°ø
ĘŚÄ©ŗĆ³É¼ØĻµĮŠ“š°ø 99¼Ó1ĮģĻČĘŚÄ©ĢŲѵ¾ķĻµĮŠ“š°ø
99¼Ó1ĮģĻČĘŚÄ©ĢŲѵ¾ķĻµĮŠ“š°ø °ŁĒæĆūŠ£ĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø
°ŁĒæĆūŠ£ĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø ŗĆ³É¼Ø1¼Ó1ĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø
ŗĆ³É¼Ø1¼Ó1ĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| FeBr3 |
| FeBr3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| FeBr3 |
| FeBr3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğ½ĖÕŹ”ŗ£ĆÅ֊ѧøßŅ»ĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø10·Ö£©Ä³æĪĶāŃŠ¾æŠŌѧĻ°Š”×éÓĆĻĀĶ¼ĖłŹ¾×°ÖĆÖʱøÉŁĮæäå±½²¢ŃéÖ¤äåÓė±½µÄ·“Ó¦ŹĒČ”“ś·“Ó¦”£
ŹµŃ鏱£¬¹Ų±ÕF»īČū£¬“ņæŖC»īČū£¬ŌŚ×°ÓŠÉŁĮæ±½µÄČżæŚÉÕĘæÖŠÓÉAæŚ¼ÓČėÉŁĮæŅŗä壬ŌŁ¼ÓČėÉŁĮæĢśŠ¼£¬Čū×”AæŚ”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£© DŹŌ¹Ü ČװµÄŹĒ £¬Ęä×÷ÓĆŹĒ ”£
ČװµÄŹĒ £¬Ęä×÷ÓĆŹĒ ”£
£Ø2£© EŹŌ¹ÜČװµÄŹĒ ”£
£Ø3£©³żČ„äå±½ÖŠ»ģÓŠµÄBr2ŌÓÖŹµÄŹŌ¼ĮŹĒ £¬²Ł×÷·½·ØĪŖ ”£
£Ø4£©ČżæŚÉÕĘæÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
£Ø5£©øł¾ŻŹ²Ć“ĻÖĻóæÉÖ¤Ć÷ŃéÖ¤äåÓė±½µÄ·“Ó¦ŹĒČ”“ś·“Ó¦£æ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģ½ĖÕŹ”øßŅ»ĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø10·Ö£©Ä³æĪĶāŃŠ¾æŠŌѧĻ°Š”×éÓĆĻĀĶ¼ĖłŹ¾×°ÖĆÖʱøÉŁĮæäå±½²¢ŃéÖ¤äåÓė±½µÄ·“Ó¦ŹĒČ”“ś·“Ó¦”£

ŹµŃ鏱£¬¹Ų±ÕF»īČū£¬“ņæŖC»īČū£¬ŌŚ×°ÓŠÉŁĮæ±½µÄČżæŚÉÕĘæÖŠÓÉAæŚ¼ÓČėÉŁĮæŅŗä壬ŌŁ¼ÓČėÉŁĮæĢśŠ¼£¬Čū×”AæŚ”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£© DŹŌ¹ÜČװµÄŹĒ £¬Ęä×÷ÓĆŹĒ ”£
£Ø2£© EŹŌ¹ÜČװµÄŹĒ ”£
£Ø3£© ³żČ„äå±½ÖŠ»ģÓŠµÄBr2ŌÓÖŹµÄŹŌ¼ĮŹĒ £¬²Ł×÷·½·ØĪŖ ”£
£Ø4£© ČżæŚÉÕĘæÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
£Ø5£© øł¾ŻŹ²Ć“ĻÖĻóæÉÖ¤Ć÷ŃéÖ¤äåÓė±½µÄ·“Ó¦ŹĒČ”“ś·“Ó¦£æ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com