
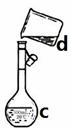
| A����30mLˮϴ���ձ�2~3�Σ�ϴ��Һ��ע������ƿ���ڲ��������в���ʧ���Һ�壬�����ʹ��Һ��Ũ��ƫ����ͣ� |
| B��ȷ��ȡ���������������ƹ������ձ��У��ټ�������ˮ��Լ30mL����������������ʹ�����ܽ� |
| C�����ܽ������������Һ�ز�����ע��mL������ƿ�� |
| D��������ƿ�ǽ�����ҡ�ȣ���ת�����Լ�ƿ�У����ϱ�ǩ�� |


 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����״���£�22.4LH2O����ԭ�Ӹ���Ϊ3NA |
| B��1.7g��������ԭ����ĿΪ0.4NA |
| C��ͬ��ͬѹʱ����ͬ������κ����嵥��������ԭ������ͬ |
| D��1 L 1mol��L-1��Na2SO4��Һ�к�Na+�ĸ���ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��6.02��1023�ǰ����ӵ������Ľ���ֵ | B�������ӵ³������������ʵ�����1mol |
| C�������Ħ��������98�� | D��1mol 12Cԭ�ӵ�����Ϊ12g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1.5molʯī����C-C����Ϊ4.5NA |
| B�����³�ѹ�£�46g��NO2��N2O4������庬�е�ԭ����Ϊ3 NA |
| C�����³�ѹ�£�22.4L��NO2��CO2�����庬��2 NA ��O ԭ�� |
| D����״���£�44.8L�������к��з�ԭ�ӵ���ĿΪ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����״����1mol H2O | B��20�桢101 kPaʱ36.5g HCl |
| C�����³�ѹ��17g NH3 | D����״����0.4 mol H2��0.6mol O2�Ļ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1��1 | B��1��4 | C��4��1 | D��1��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com