
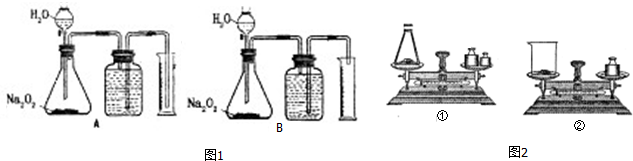
���� ��1��500mL��Ͳ��ȡ���ǹ���������ˮ��Ӧ�������������������500mL�������������������Ƶ����������
��2�����������������տ����е�H2O��CO2�����˱�¶�ڿ����г������ձ��й�������������Ӵ������
��3����Na2O2��ˮ��Ӧ����ȶ�ʹ�����������������¹������Ƶ���������ƫ��Ӧ��ȴ���壬ѡ�ó����ܣ�
��4����Ͳ���ռ�����Һ�������ΪO2������������������������������Ƶ�����
��� �⣺��1��500mL��Ͳ��ȡ���ǹ���������ˮ��Ӧ������������������ɵ�500mL��������ԼΪ0.022mol������2Na2O2+2H2O=4NaOH+O2������Ҫ������������ԼΪ0.044mol��78g/mol=3.4g��Na2O2������ӦС��3.4g���������������Ͳ��Һ�����磬������ٲ���������̫�٣�������������Ͳ�����������̫��ѡ��Na2O2��Ʒ�ĺ�������Ϊ2.5g��3.0g��
�ʴ�Ϊ��B��
��2�����������������տ����е�H2O��CO2�����˱�¶�ڿ����г������ձ��й�������������Ӵ����������ѡ������ƿ��ȡ��
�ʴ�Ϊ���٣����������������տ����е�H2O��CO2�����˱�¶�ڿ����г�����
��3����Na2O2��ˮ��Ӧ����ȶ�ʹ�����������������¹������Ƶ���������ƫ��Ӧ��ȴ���壬ѡ�ó����ܣ���ֹ��Ͳ�Ķ�����������ʱ�ų��������������
�ʴ�Ϊ��A��ƫ��
��4����Ͳ���ռ�����Һ�������ΪO2������������ʵ���n��O2��=$\frac{{10}^{-3}V}{a}$mol�����ݷ�Ӧ��ϵʽ��2Na2O2��O2�����ɵ�n��Na2O2��=2��$\frac{{10}^{-3}V}{a}$mol��Na2O2����������=$\frac{2��\frac{{10}^{-3}V}{a}mol��78g/mol}{mg}$��100%=$\frac{15.6V}{am}$%��
�ʴ�Ϊ��$\frac{15.6V}{am}$%��
���� ���⿼���˹���������ˮ��Ӧ������ʵ���Լ��ⶨ���ʵ�����������ʵ�飬��Ŀ��Ϊ�ۺϣ��ѶȲ���ע�����ʵ�鷽�������ԭ����


 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ��pH���������� | |
| B�� | ��������п�۷�Ӧ���������������ͬ | |
| C�� | ������п����Ӧʱ��һ��ʼ��������ʿ� | |
| D�� | ���к�NaOH��Һ�����ʵ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| Ԫ�� | Mn | Fe | |
| �����ܣ�KJ•mol-1�� | ��1 | 717 | 759 |
| ��2 | 1509 | 1561 | |
| ��3 | 3248 | 2957 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| Ũ��/mol•L-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����Ƕ���ֲ��������������Ҫ��Դ����֬�Dz���������ߵ�Ӫ������ | |
| B�� | ��ʯȼ�ϵĴ���ʹ���Dz���PM2.5����Ҫԭ��֮һ | |
| C�� | ̼�����������Ҳ���մ�������ϴ�����þߵ����� | |
| D�� | ��ͭ���ҹ�ʹ������ĺϽ���ϣ�Ŀǰ������ʹ�������ĺϽ���������Ͻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
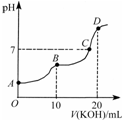
| A�� | KHC2O4��Һ�������� | |
| B�� | B��ʱ��c��HC2O4-����c��K+����c��H+����c��OH-�� | |
| C�� | C��ʱ��c��HC2O4-��+c��C2O42-��+c��H2C2O4����c��K+����c��HC2O4-��+2c��C2O42-��+c��H2C2O4�� | |
| D�� | D��ʱ��c��H+��+c��HC2O4-��+c��H2C2O4��=c��OH-�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com