
����Լռ����������71%����ˮ��ѧ��Դ�����þ��зdz�������ǰ����
��1����ˮɹ�οɻ�ô��Σ���ʵ�����д��ξ����ܽ⡢ �� ���Ƶþ��Ρ�
��2��þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ģ�����Ҫ�������£�
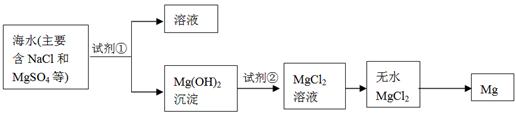
��Ϊ��ʹMgSO4ת��ΪMg(OH) 2���Լ��ٿ���ѡ�� ��ҪʹMgSO4��ȫת��Ϊ�����������Լ��ٵ���Ӧ ��
���Լ��ڿ���ѡ�� ��
���Դӽ�Լ��Դ����߽���þ�Ĵ��ȷ������������˵�ұþ������ ��

��3���弰�仯������;ʮ�ֹ㷺���ҹ����ڴ�����չ��ˮ������о��Ϳ�����������ҵ��Ũ����ˮΪԭ����ȡ��IJ��ֹ������£�
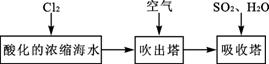
ij����С����ʵ����ģ�����������������װ�ý���ʵ�飨��������Ʒ���ѱ��������г�װ������ȥ����
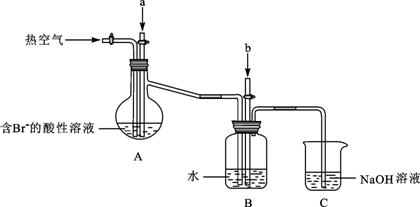
��Aװ����ͨ��a�����Ŀ���ǣ������ӷ���ʽ��ʾ�� ��
��Aװ����ͨ��a����һ��ʱ���ֹͣͨ�룬��ͨ�ȿ�����ͨ���ȿ�����Ŀ����
��
�۷�Ӧ�����У�Bװ������SO42-���ɡ�����SO42-�ķ����� ��
��Cװ�õ������� ��
��1�����ˡ����� ............................................................��2�֣�
��2�����������ƣ�NaOH�� ���� ............................��2�֣�
������ .............................................................��1�֣�
�� C ............................................................��1�֣�
��3���� Cl2+2Br- =2Cl- +Br2 ................................................��2�֣�
�� ����Br2 .............................................................��1�֣�
��ȡ����Bװ���з�Ӧ�����Һ���Թ��У��μ��Ȼ�����Һ��������ɫ������֤����SO42- .............................................................��1�֣�
�� ����δ��Ӧ��Cl2��Br2��SO2 .............................��1�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ���� |

| ���� |


| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?������һģ������Լռ����������71%��������п������õIJ���������ͼ��ʾ������˵������ȷ���ǣ�������
��2012?������һģ������Լռ����������71%��������п������õIJ���������ͼ��ʾ������˵������ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� |
| ||
| 2800�� |
| �� |
| ||
| ��ԭ |
| HCl |
| ||
| 714�� |
| HCl |
| ||
| ��ԭ |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �� |
| ��� |
| 2800�� |
| �� |
| C |
| ��ԭ |
| HCl |
| ||
| 714�� |
| HCl |
| ���ý��� |
| ��ԭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
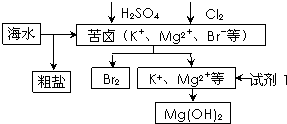
| A������BaCl2��Һ��ȥ�����е�SO42- | B���ӿ�±����ȡBr2�ķ�Ӧ�����ӷ���ʽΪ��2Br-+Cl2�T2Cl-+Br2 | C���Լ�1����ѡ��ʯ���� | D����ҵ�ϣ��������MgOұ������þ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com