
��һ����ɫ��ĩ�����п��ܺ���Ba(NO3)2��CaCl2��K2CO3��������ʵ�飺
��1���������ַ�ĩ����ˮ�У����а�ɫ�������ɡ�
��2����1��������Һ�м��������ϡ���ᣬ��ɫ������ʧ���������ݲ�����
��3��ȡ������2������Һ����ϡ���ᣬ�а�ɫ�������ɡ�
��4����ȡ������2������Һ������������Һ���а�ɫ�������ɡ�
�Իش�
��1������������ʵ�������ж�ԭ��ɫ��ĩ����ɳɷ��ǣ�д���ƣ�
��2��д��ʵ�鲽�裨1���ͣ�3�����йػ�ѧ��Ӧ�Ļ�ѧ����ʽ
��
��
��
 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ����ɫ��ĩ�����п��ܺ���Ba(NO3)2��CaCl2��K2CO3����������ʵ�飺
�ٽ����ַ�ĩ����ˮ�У����а�ɫ�������ɡ�
����ٵ�����Һ�м������ϡ���ᣬ��ɫ������ʧ���������ݲ�����
��ȡ�����ڵ���Һ���μ�ϡ���ᣬ�а�ɫ�������ɡ�
����ȡ�����ڵ���Һ���μ�AgNO3��Һ���а�ɫ�������ɡ�
��ش�
��1����������ʵ�������ж�ԭ��ɫ��ĩ����ɳɷ���___________����д���ƣ���
��2��д���١������漰�Ļ�ѧ����ʽ��
��______________________________
��_____________________________
��______________________________
��_____________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ����һ�и߶���ѧ�����п��Ի�ѧ���ģ��Ծ� ���ͣ������
(8��)��һ����ɫ��ĩ�����п��ܺ���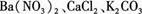 ����������ʵ�顣
����������ʵ�顣
(1)�����ַ�ĩ����ˮ�У����а�ɫ�������ɣ�
(2)��(1)������Һ�м������ϡ���ᣬ��ɫ������ʧ���������ݲ�����
(3)ȡ����(2)����Һ����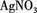 ��Һ���а�ɫ�������ɡ�
��Һ���а�ɫ�������ɡ�
��������ʵ�� �����жϰ�ɫ��ĩ��һ������ (�ѧʽ)�����ܺ��� (�ѧʽ)��д��(3)���йط�Ӧ�����ӷ���ʽ ��
�����жϰ�ɫ��ĩ��һ������ (�ѧʽ)�����ܺ��� (�ѧʽ)��д��(3)���йط�Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com