
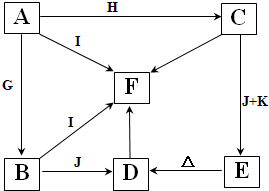
 A-K����ѧ��ѧ�г����ļ������ʣ�����֮���ת����ϵ��ͼ��ʾ����֪������AΪ���嵥�ʣ�BΪ����ɫ��ĩ��G��HΪ��̬���ʣ�I�ڳ�����ΪҺ�壬D��E��F��ˮ��Һ���ʼ��ԣ���C�ı�����Һ��ȡF��E����Ҫ�Ļ����������ش��������⣺
A-K����ѧ��ѧ�г����ļ������ʣ�����֮���ת����ϵ��ͼ��ʾ����֪������AΪ���嵥�ʣ�BΪ����ɫ��ĩ��G��HΪ��̬���ʣ�I�ڳ�����ΪҺ�壬D��E��F��ˮ��Һ���ʼ��ԣ���C�ı�����Һ��ȡF��E����Ҫ�Ļ����������ش��������⣺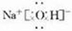 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� Cl2��+H2��+2NaOH������·��ͨ��0.1mol����ʱ������NaOH0.1mol������Һ��pHΪ13���ʴ�Ϊ��13��
Cl2��+H2��+2NaOH������·��ͨ��0.1mol����ʱ������NaOH0.1mol������Һ��pHΪ13���ʴ�Ϊ��13��

 ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ��Ӫ��ʤ���ڶ���ѧ2011�������ѧ������ѧ�ο��Ի�ѧ���� ���ͣ�022
(��)��ѧʵ���У�������Һ���Լ������ữ�������ữ�����Ĵ�ʩ����ȷ����________
A�����Լ���SO32��������HNO3�ữ��BaCl2��Һ
B������FeCl2��Һʱͨ��������HNO3�ữ����С��ˮ��̶�
C������ij��Һ���Ƿ�Cl������HNO3�ữ��AgNO3��Һ
(��)���ӷ�Ӧ����ѧ��ѧ����Ҫ�ķ�Ӧ���ͣ��ش��������⣺
(1)�ڷ������ӷ�Ӧ�ķ�Ӧ����������У�һ��������________(����)��
�ٵ���
��������
�۵����
����
�ݻ�����
(2)һ����ɫ����Һ��
�����ܺ�������������K+��Mg2+��Al3+��Fe2+��Ba2+��NO3����SO42����HSO3����HCO3����I����Cl����ȡ����Һ��������ʵ�����ٽ���Һ������ɫʯ����ֽ�ϣ��ʺ�ɫ��
�ڽ�������ҺŨ�������ͭƬ�����ᣬ����ɫ����������������ͨ������������ɺ���ɫ��
��ȡ������Һ����BaCl2��Һ���������ɫ������
��ȡʵ����еij�����Һ������AgNO3��Һ������������ϡHNO3�İ�ɫ������
����ȡ������Һ������NaOH��Һ���а�ɫ�������ɣ���NaOH����ʱ�����в��ְ�ɫ�����ܽ⣮
�������������жϣ�ԭ��Һ�п϶������ڵ�������________���϶����ڵ�������________�����������жϵ�������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ����һ�и����ڶ����¿����ۺϲ��ԣ���ѧ���֣� ���ͣ������
��16�֣���ͼ����ѧ�������ʼ��ת����ϵ����֪��
a��AΪ����ɫ���壬BΪ���¡�����ЧӦ������Ҫ���ʣ�
b��EΪ����������JΪ���ɫ������
c��G��ʵ�����г����ڼ���B�Ĵ��ڣ�
d��L��һ����Ҫ�Ĺ�ҵԭ�ϣ�����������ըҩ��Ũ��Һ�����治�����ʻ�ɫ��
�ش��������⣺
��1��A�ĵ���ʽΪ ��B�������� ���ӣ����ԡ��Ǽ��ԣ���
��2����Ӧ�ٵĻ�ѧ����ʽΪ ��
��Ӧ�ڵ����ӷ���ʽΪ ��
��3�����μӷ�Ӧ��A������Ϊ39g��������CO2�����������£�Ϊ L��
��4������K�������ӳ��õķ����� ��
��5��LŨ��Һ�ı��淽���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�챱���к�����������ѧ��������ϰ��ѧ�Ծ� ���ͣ������
��12�֣���֪��A��B��CΪ��ѧ�����ĵ��ʣ�AΪ����ɫ���壻D��E��FΪ��ѧ�����������E�Ǿ��д��Եĺ�ɫ���壻H��KΪ��ѧ�������Σ�MΪһ�ֳ�������ɫҺ�塣�����ʼ��ת����ϵ����ͼ��ʾ��ijЩ��������ȥ����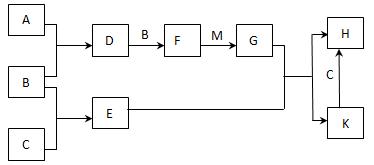
��ش�
��1������K�Ļ�ѧʽΪ��������������
��2������M�ĵ���ʽΪ��������������
��3��D��ʹ����KMnO4��Һ��ɫ��������D���ʵ���������������ĸ��ţ���
A. �����ԡ����� B. ��ԭ�ԡ����� C. Ư����
��4��C��M��һ�������·�Ӧ�Ļ�ѧ����ʽΪ ��
��5���Ƚ�D����ͨ��BaCl2��Һ�У���ͨ��NH3��ʵ������е�����Ϊ�� ������������ ��
��6��H��Һ�ڿ����г��ڷ��û���ֺ��ɫ���ǣ�����һ�����ӷ���ʽ��ʾ��仯��ԭ�� ��
��7��A��C������һ�������·������Ϸ�Ӧ���������Ԫ��A��C��������Ϊ4��7����������ij��ʯ����Ҫ�ɷ�֮һ������������G��ij���������ÿ�ʯ��Ʒ������25%����ȡ8.8�ָÿ�ʯ������������������������ʧ�����Ƶú�G 98%�IJ�Ʒ �֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
����2�֣����в����ᵼ��ʵ����ƫ�ߵ���
A������һ�����ʵ���Ũ�ȵ�������Һʱ������ҡ�Ⱥ���Һ����ڿ̶��ߡ�
B������һ�����ʵ���Ũ����Һʱ������ʱ���Ӷ�����������Һ��Ũ��
C������ƽ����20.5gij���ʣ������ҩƷ��λ�÷ŷ�������ҩƷ������
D��10%�������90%�����������������50����������Һ
����2�֣���ѧʵ���У�������Һ���Լ������ữ�������ữ�����Ĵ�ʩ����ȷ����
A.���Լ���SO32-������HNO3�ữ��BaCl2��Һ
B. ����FeCl2��Һʱͨ��������HNO3�ữ����С��ˮ��̶�
C.����ij��Һ���Ƿ�Cl-����HNO3�ữ��AgNO3��Һ
(��)�����ӷ�Ӧ����ѧ��ѧ����Ҫ�ķ�Ӧ���ͣ��ش��������⣺
��1����2�֣��ڷ������ӷ�Ӧ�ķ�Ӧ����������У�һ�������� �����ţ���
�ٵ��� �������� �۵���� ���� �ݻ�����
��2����6�֣�һ����ɫ����Һ�У����ܺ�����������:K����Mg2����Al3����Fe2����Ba2����NO3����SO42����HSO3����HCO3����I����Cl����ȡ����Һ��������ʵ�飺
�ٽ���Һ������ɫʯ����ֽ�ϣ��ʺ�ɫ��
�ڽ�������ҺŨ�������ͭƬ�����ᣬ����ɫ����������������ͨ������������ɺ���ɫ��
��ȡ������Һ����BaCl2��Һ���������ɫ������
��ȡʵ����еij�����Һ������AgNO3��Һ������������ϡHNO3�İ�ɫ������
����ȡ������Һ������NaOH��Һ���а�ɫ�������ɣ���NaOH����ʱ�����в��ְ�ɫ�����ܽ⡣
�������������жϣ�ԭ��Һ�п϶������ڵ������� ���϶����ڵ������� �����������жϵ������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com