
½öÓĆĻĀ±ķĢį¹©µÄŹµŃé²Ł×÷»ņ²£Į§ŅĒĘ÷(·Ē²£Į§ŅĒĘ÷ČĪŃ”)¾ĶÄܹ»“ļµ½ĻąÓ¦ŹµŃéÄæµÄµÄŹĒ(””””)
| Ń”Ļī | ŹµŃé²Ł×÷»ņ²£Į§ŅĒĘ÷ | ŹµŃéÄæµÄ |
| A | ĖįŹ½µĪ¶Ø¹Ü”¢¼īŹ½µĪ¶Ø¹Ü”¢ÉÕ±”¢×¶ŠĪĘæ | ÓĆ0.01 mol/LµÄĻ”ŃĪĖį±ź¶ØĪ“ÖŖNaOHČÜŅŗµÄÅØ¶Č |
| B | ·ÖŅŗĀ©¶·”¢×¶ŠĪĘ攢øÉŌļ¹Ü”¢µ¼¹Ü”¢¼ÆĘųĘæ | ÓĆ¼īŹÆ»ŅŗĶÅØ°±Ė®ÖʱøÉŁĮæNH3 |
| C | ½«ÅØĮņĖįŗĶĢ¼»ģŗĻ¼ÓČČ£¬Ö±½Ó½«Éś³ÉµÄĘųĢåĶØČė×ćĮæµÄŹÆ»ŅĖ®ÖŠ£¬ŹÆ»ŅĖ®±ä»ė×Ē | ¼ģŃéĘųĢå²śĪļÖŠCO2µÄ“ęŌŚ |
| D | ĻņijČÜŅŗÖŠ¼ÓČėBaCl2ČÜŅŗ£¬²śÉś°×É«³Įµķ | ¼ģŃéSO |
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»š¼żĶĘ½ųĘ÷ÖŠŹ¢ÓŠĒ滹Ō¼ĮŅŗĢ¬ėĀ£ØN2H4£©ŗĶĒæŃõ»Æ¼ĮŅŗĢ¬Ė«ŃõĖ®”£µ±°Ń0.4molŅŗĢ¬ėĀŗĶ0.8molŅŗĢ¬ H2O2»ģŗĻĒ”ŗĆĶźČ«·“Ó¦£¬Éś³ÉµŖĘųŗĶĖ®ÕōĘų£¬·Å³ö256 kJµÄČČĮ攣
£Ø1£©·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ÓÖŅŃÖŖ16gŅŗĢ¬ėĀÓėŅŗĢ¬Ė«ŃõĖ®·“Ӧɜ³ÉŅŗĢ¬Ė®Ź±·Å³öµÄČČĮæŹĒ408 kJ”£ŌņH2O(g)£½H2O(l)
µÄ¦¤H£½ ”£
(3)ŅŃÖŖN2(g) + 2O2(g) == 2NO2(g)£»”÷H=+67.7KJ/mol£¬
N2H4(g) + O2(g) == N2(g)+2H2O(g)£»”÷H= ”Ŗ534KJ/mol£¬
ŌņėĀÓėNO2ĶźČ«·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ_____ _____________________”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆŗĻĪļKxFe(C2O4)y”¤3H2O(FeĪŖ£«3¼Ū)ŹĒŅ»ÖÖ¹āĆō²ÄĮĻ£¬ŹµŃéŹŅæÉŅŌÓĆČēĻĀ·½·ØĄ“ÖʱøÕāÖÖ²ÄĮĻ²¢²ā¶ØÕāÖÖ²ÄĮĻµÄ×é³É£ŗ
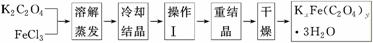
(1)½į¾§Ź±Ó¦½«ČÜŅŗÓƱłĖ®ĄäČ“ÖĆÓŚŌŚŗŚ°µ“¦µČ“ż¾§ĢåµÄĪö³ö£¬ÕāŃł²Ł×÷µÄŌŅņŹĒ£ŗ________________________________________________________________________”£
(2)²Ł×÷¢ńµÄĆū³ĘŹĒ______________________”£[Ą“Ō“:ѧæĘĶų]
(3)³ĘČ”Ņ»¶ØÖŹĮæµÄ¾§ĢåÖĆӌ׶ŠĪĘæÖŠ£¬¼ÓČė×ćĮæµÄÕōĮóĖ®ŗĶĻ”H2SO4£¬½«C2O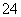 ×Ŗ»ÆĪŖH2C2O4ŗóÓĆ0.100 0 mol”¤L£1KMnO4ČÜŅŗµĪ¶Ø£¬µ±ĻūŗÄKMnO4ČÜŅŗ24.00 mLŹ±Ē”ŗĆĶźČ«·“Ó¦£¬H2C2O4ÓėĖįŠŌKMnO4ČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ__________________________”£ŌŁĻņČÜŅŗÖŠ¼ÓČėŹŹĮæµÄ»¹Ō¼Į£¬Ē”ŗĆ½«Fe3£«ĶźČ«×Ŗ»ÆĪŖFe2£«£¬ÓĆKMnO4ČÜŅŗ¼ĢŠųµĪ¶Ø”£µ±Fe2£«ĶźČ«Ńõ»ÆŹ±£¬ÓĆČ„KMnO4ČÜŅŗ4.00 mL£¬“ĖµĪ¶Ø·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ____________”£
×Ŗ»ÆĪŖH2C2O4ŗóÓĆ0.100 0 mol”¤L£1KMnO4ČÜŅŗµĪ¶Ø£¬µ±ĻūŗÄKMnO4ČÜŅŗ24.00 mLŹ±Ē”ŗĆĶźČ«·“Ó¦£¬H2C2O4ÓėĖįŠŌKMnO4ČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ__________________________”£ŌŁĻņČÜŅŗÖŠ¼ÓČėŹŹĮæµÄ»¹Ō¼Į£¬Ē”ŗĆ½«Fe3£«ĶźČ«×Ŗ»ÆĪŖFe2£«£¬ÓĆKMnO4ČÜŅŗ¼ĢŠųµĪ¶Ø”£µ±Fe2£«ĶźČ«Ńõ»ÆŹ±£¬ÓĆČ„KMnO4ČÜŅŗ4.00 mL£¬“ĖµĪ¶Ø·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ____________”£
(4)ÅäÖĘ100 mL 0.100 0 mol”¤L£1KMnO4ČÜŅŗ¼°µĪ¶ØŹµŃéÖŠĖłŠčµÄ²£Į§ŅĒĘ÷³żÉÕ±”¢²£Į§°ō”¢½ŗĶ·µĪ¹Ü”¢ĮæĶ²”¢×¶ŠĪĘæĶā»¹ÓŠ________(ĢīŅĒĘ÷Ćū³Ę)”£µĪ¶ØÖÕµćŹ±ČÜŅŗŃÕÉ«ĪŖ________É«£¬ĒŅ30ĆėÄŚ²»±äÉ«”£
(5)¾¼ĘĖć£¬»ÆŗĻĪļKxFe(C2O4)y”¤3H2OÖŠ£¬x£½________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓĆČēĶ¼ĖłŹ¾ŹµŃé×°ÖĆ½ųŠŠĪļÖŹŠŌÖŹµÄĢ½¾æŹµŃ锣ĻĀĮŠĖµ·Ø²»ŗĻĄķµÄŹĒ(””””)

A£®ČōĘ·ŗģČÜŅŗ¢ŁĶŹÉ«£¬ŌņĖµĆ÷²śĪļÖŠŗ¬ÓŠSO2
B£®ČōäåĖ®ĶŹÉ«£¬ŌņĖµĆ÷SO2¾ßÓŠ»¹ŌŠŌ
C£®ČōÉÕĘæÖŠ²śÉś»ĘÉ«»ė×ĒŗĶĪŽÉ«ĘųÅŻ£¬ŌņĖµĆ÷Na2S2O3Ö»×÷Ńõ»Æ¼Į
D£®ČōĘ·ŗģČÜŅŗ¢Ś²»ĶŹÉ«”¢Na2SiO3ČÜŅŗÖŠ³öĻÖ°×É«»ė×Ē£¬ŌņĖµĆ÷ŃĒĮņĖį±ČĢ¼ĖįµÄĖįŠŌĒæ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
“óĘųÖŠĮņ”¢µŖµÄŃõ»ÆĪļŹĒŠĪ³ÉĖįÓźµÄÖ÷ŅŖĪļÖŹ”£Ä³µŲĖįÓźÖŠæÉÄÜŗ¬ÓŠĻĀĮŠĄė×Ó£ŗNa£«”¢Ba2£«”¢NH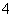 ”¢Al3£«”¢Cl£”¢SO
”¢Al3£«”¢Cl£”¢SO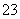 ”¢SO
ӢSO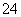 ӢNO
”¢NO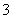 µČ”£Ä³ŃŠ¾æŠ”×éČ”øƵŲŅ»¶ØĮæµÄĖįÓź£¬ÅØĖõŗó½«ĖłµĆ³ĪĒåŹŌŅŗ·Ö³ÉČż·Ż£¬½ųŠŠČēĻĀŹµŃé£ŗ
µČ”£Ä³ŃŠ¾æŠ”×éČ”øƵŲŅ»¶ØĮæµÄĖįÓź£¬ÅØĖõŗó½«ĖłµĆ³ĪĒåŹŌŅŗ·Ö³ÉČż·Ż£¬½ųŠŠČēĻĀŹµŃé£ŗ
| ŹŌŃł | Ėł¼ÓŹŌ¼Į | ŹµŃéĻÖĻó |
| µŚŅ»·ŻŹŌŅŗ | µĪ¼ÓŹŹĮæµÄµķ·ŪKIČÜŅŗ | ČÜŅŗ³ŹĄ¶É« |
| µŚ¶ž·ŻŹŌŅŗ | µĪ¼ÓÓĆŃĪĖįĖį»ÆµÄBaCl2ČÜŅŗ | ÓŠ°×É«³Įµķ²śÉś |
| µŚČż·ŻŹŌŅŗ | µĪ¼ÓNaOHČÜŅŗ£¬¼ÓČČ£¬¼ÓČėµÄNaOHČÜŅŗĢå»ż(V)ÓėÉś³ÉµÄ³Įµķ”¢²śÉśµÄĘųĢåµÄĪļÖŹµÄĮæ(n)µÄ¹ŲĻµČēÓŅĶ¼ |
|
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)øł¾ŻŹµŃé½į¹ūÅŠ¶ĻøĆĖįÓźÖŠæĻ¶Ø²»“ęŌŚµÄĄė×ÓŹĒ______________£¬²»ÄÜČ·¶ØµÄĄė×ÓÓŠ________________”£
(2)Š“³öµŚŅ»·ŻŹŌŅŗµĪ¼Óµķ·ŪKIČÜŅŗŹ±·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ__________________”£
(3)µŚČż·ŻŹŌŅŗµĪ¼ÓNaOHČÜŅŗ£¬¼ÓČČ£¬Õūøö¹ż³ĢÖŠ·¢ÉśĮĖ¶ąøö·“Ó¦£¬Š“³öĘäÖŠĮ½øö·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ__________________________________”¢__________________________”£
(4)Éč¼ĘŹµŃé·½°ø£¬¼ģŃéøĆĖįÓźÖŠŹĒ·ń“ęŌŚCl££ŗ___________________________________
______________________________ӣ
(5)øĆŠ”×éĪŖĮĖĢ½¾æNO²ĪÓėĮņĖįŠĶĖįÓźµÄŠĪ³É¹ż³Ģ£¬ŌŚÉÕĘæÖŠ³äČėŗ¬ÓŠÉŁĮæNOµÄSO2ĘųĢ壬ŌŁĀżĀżĶØČėO2£¬·¢Éś»Æѧ·“Ó¦ŗó£¬ŌŁÅēČ÷ŹŹĮæÕōĮóĖ®¼“µĆĮņĖįŠĶĖįÓź£¬ŌņNOŌŚÉĻ Źö·“Ó¦ÖŠµÄ×÷ÓĆŹĒ________________________________________________________”£
Źö·“Ó¦ÖŠµÄ×÷ÓĆŹĒ________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij»ģŗĻĘųĢåX£¬æÉÄÜÓÉH2”¢CO”¢CO2ŗĶĖ®ÕōĘųÖŠµÄŅ»ÖÖ»ņ¼øÖÖ×é³É£¬ĻÖ½«»ģŗĻĘųĢåĶØČė×ĘČȵÄCuO£¬ĶźČ«·“Ó¦ŗ󣬹ĢĢåCuOÖŹĮæ¼õÉŁ1.6 g£»ŌŁ½«·“Ó¦ŗóµÄ»ģŗĻĘųĢåČ«²æµ¼Čė×ćĮæµÄ³ĪĒåŹÆ»ŅĖ®ÖŠ£¬Éś³É°×É«³Įµķ10 g”£øł¾ŻŅŌÉĻŠÅĻ¢£¬·ÖĪöĻĀĮŠÓŠ¹ŲXµÄ×é³ÉĶʶĻÕżČ·µÄŹĒ(””””)
A£®XŅ»¶ØÖ»ÓÉCO×é³É
B£®XŅ»¶ØÖ»ÓÉH2ŗĶCO2×é³É
C£®XæÉÄÜÓÉ0.1 g H2ŗĶ4.4 g CO2×é³É
D£®XæÉÄÜÓÉ0.1 g H2”¢1.4 g CO”¢2.2 g CO2×é³É
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĀ±“śĢžŌŚKOH“¼ČÜŅŗÖŠ¼ÓČČ²»ÄÜ·¢ÉśĻūČ„·“Ó¦µÄŹĒ
(”””” )
¢ŁC6H5Cl””¢Ś(CH3)2CHCH2Cl””¢Ū(CH3)3CCH2Cl
¢ÜCHCl2”ŖCHBr2””¢ŻCH3CH2Br””¢ŽCH2Cl2
A£®¢Ł¢Ū¢Ž B£®¢Ś¢Ū¢Ż
C£®Č«²æ D£®¢Ś¢Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ·“Ó¦ŹōÓŚĻūČ„·“Ó¦µÄŹĒ
(””””)
A£®ŅŅ“¼ÓėÅØĮņĖį¹²Čȵ½140 ”ę
B£®ŅŅ“¼ÓėŃõĘų·“Ӧɜ³ÉŅŅČ©
C£®ŅŅ“¼Č¼ÉÕÉś³É¶žŃõ»ÆĢ¼ŗĶĖ®
D£®ŅŅ“¼ÓėÅØĮņĖį¹²Čȵ½170 ”ę
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚĻĀĮŠĪļÖŹÖŠ·Ö±š¼ÓČė½šŹōÄĘ£¬²»ÄܲśÉśĒāĘųµÄŹĒ
(”””” )
A£®ÕōĮóĖ®”””””””””””””” B£®ĪŽĖ®¾Ę¾«
C£®±½ D£®75%µÄ¾Ę¾«
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com