
�屽��һ�ֳ��õĻ���ԭ�ϡ�ʵ�����Ʊ��屽
��ʵ�鲽�����£�
����1����a�м���15 mL����������м���ٽ�b��4.0 mL
Һ���������뵽a�У���ַ�Ӧ��
����2����a�м���10 mLˮ��Ȼ����˳�ȥδ��Ӧ����м���߿���Դ��
����3����Һ������10 mLˮ��8 mL 10%��NaOH��Һ��10 mL ˮϴ�ӣ���Һ�ô��屽��
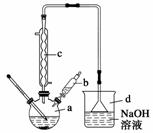 ����4����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����˼��ôֲ�Ʒ��
����4����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����˼��ôֲ�Ʒ��
| �� | �� | �屽 | |
| �ܶ�/g��cm��3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ��ˮ�е��ܽ�� | �� | �� | �� |
(1)����d�������� ��
(2)��b�е�Һ���������뵽a�У������ܿ��ټ����ԭ���� ��
(3)����c��������������������������Ҫ������ (�ѧʽ)��
(4)����4�õ��Ĵֲ�Ʒ�л��������ʱ�����֪�����屽���й������������ϱ�����Ҫ��һ���ᴿ�ֲ�Ʒ����������е�ʵ����������� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й����л��������˵����ȷ���� �� ��
A�������������������Na2CO3��Һ��������
B�����飨C5H12��������ͬ���칹��
C����ϩ��������ϩ�ͱ������о�����̼̼˫��
D�����ࡢ��֬�͵����ʾ��ɷ���ˮ�ⷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ�ڿ�ѧʵ���о�����Ҫ�����ã�����������ʵļ���ͷ����ᴿ����6.72L����״������ϩ�ͼ���Ļ����ͨ����������ˮ�У���ַ�Ӧ����ˮ������������1.4g������ʽ����ԭ�����������ϩ�ͼ�������ʵ���֮���Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
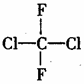
�Է��ﰺ���ṹ����ͼ�����й�������ȷ���� �� ��
A��������ͬ���칹�� B����ƽ���ͷ���
C����һ���������� D���ڸ߿տ��ƻ�������
 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���ʮ���ֻ�ѧ���ţ�
��O2 ��614C ��238U ��1123Na ��O3 ��714N ��1224Mg ��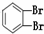
��235U ��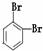 ��11��
��11��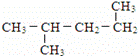 ��12��
��12��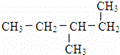
����
��1����ʾ���صķ��Ź��Уߣߣ���.
��2����Ϊͬλ�ص��ǣߣߣߣ�����ţ�������ͬ��.
��3����������ȣ������ܻ���ͬλ�ص��ǣߣߣ�.
��4����������ȣ�������������ȵ��ǣߣߣ�.
��5����Ϊͬ����������ǣߣߣ�.
��6����Ϊͬ���칹����ǣߣߣߣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й��������ʵ�˵���������
A�����ȶ��ԣ�HCl��HI B��ԭ�Ӱ뾶��Na��Mg
C�����ԣ�H2SO3��H2SO4 D����ԭ�ԣ�S2����Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ũ������H3AsO3��SnCl2�����ӷ���ʽΪ��3SnCl2��12Cl����6 H����2H3AsO3=== 2As ��3SnCl62����6M�����ڸ÷�Ӧ��˵������ȷ�������
����������H3AsO3 �ڻ�ԭ�ԣ�Cl����As �� ÿ����1mol As����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ3 mol �� MΪOH�� �� SnCl62������������
A���٢ۢ� B���٢ڢܢ� C���٢ڢۢ� D��ֻ�Т٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��̬��A��H2������ܶ�Ϊ14�������������������һ�����ҵ�ʯ�ͻ�����չˮƽ���Ըû�����Ϊԭ�Ϻϳɻ�����G��E��I���������£�
��֪�������廯����FΪC��H��O���������Է�������Ϊ166�����ϵ�һ�ȴ�����һ�֣�1 mol F������NaHCO3��Һ��Ӧ������2 mol CO2��F������B��Ӧ����G��
��HΪ��Ԫ�����������ܶ�����ɱ�״��Ϊ2.77 g/L��H������D��Ӧ����I��
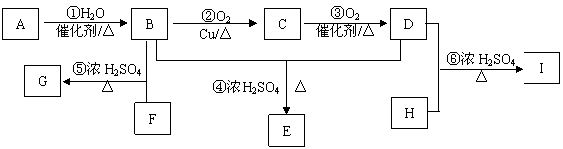
(1)A�й����ŵ�����Ϊ ��E�Ľṹ��ʽ ��
(2)G�ķ���ʽΪ ����Ӧ�ݵķ�Ӧ����Ϊ ��
(3)д�����л�ѧ����ʽ��
�� ��
�� ��
(4)F��H�����ɸ߷��ӻ�����J��д������J�Ļ�ѧ��Ӧ����ʽ��
��
(5)I�ж���ͬ���칹�壬����һ��ͬ���칹��������������
�ٷ����к�����Ԫ���ṹ����1 mol���л���������NaHCO3��Һ��Ӧ��������1 mol CO2����1 mol���л���������Na��Ӧ��������1.5 mol H2���ܻ��ϵ�һ�ȴ���ֻ�����֡������������������л�������п��ܵĽṹ��ʽΪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ɫ��Һ��SO2���ã�������ɫ���䷴Ӧʵ����ͬ���ǣ� ��
��Ʒ����Һ������KMnO4��Һ����ˮ�ܵ����̪���ռ���Һ�ݵ��ۡ�����Һ
A���٢ڢ� B���ڢۢ� C���ۢܢ� D���ڢۢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com