
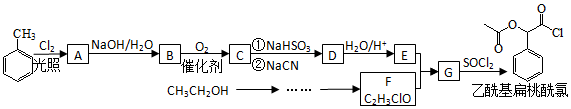
 ��RCN$\stackrel{H_{2}O/H+}{��}$RCOOH��RCOOH$\stackrel{SOCl}{��}$RCOCl$\stackrel{R��OH}{��}$RCOOR��
��RCN$\stackrel{H_{2}O/H+}{��}$RCOOH��RCOOH$\stackrel{SOCl}{��}$RCOCl$\stackrel{R��OH}{��}$RCOOR������ 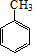 ���������º���������ȡ����Ӧ����AΪ
���������º���������ȡ����Ӧ����AΪ ��A������������Һ��ˮ������BΪ
��A������������Һ��ˮ������BΪ ��B��������Ӧ����CΪ
��B��������Ӧ����CΪ ��C��NaCN��NaHSO3����������DΪ
��C��NaCN��NaHSO3����������DΪ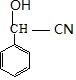 ��D������Һ��ˮ������EΪ
��D������Һ��ˮ������EΪ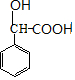 �����G��SOCl2��Ӧ�����������������ȣ��ж�FΪCH3COCl����֪��RCHO$��_{��NaCN}^{��NaHSO_{3}}$
�����G��SOCl2��Ӧ�����������������ȣ��ж�FΪCH3COCl����֪��RCHO$��_{��NaCN}^{��NaHSO_{3}}$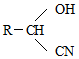 ��RCN$\stackrel{H_{2}O/H+}{��}$RCOOH��RCOOH$\stackrel{SOCl}{��}$RCOCl���ݴ���ƺϳ�·�ߣ�
��RCN$\stackrel{H_{2}O/H+}{��}$RCOOH��RCOOH$\stackrel{SOCl}{��}$RCOCl���ݴ���ƺϳ�·�ߣ�
��� �⣺ ���������º���������ȡ����Ӧ����AΪ
���������º���������ȡ����Ӧ����AΪ ��A������������Һ��ˮ������BΪ
��A������������Һ��ˮ������BΪ ��B��������Ӧ����CΪ
��B��������Ӧ����CΪ ��C��NaCN��NaHSO3����������DΪ
��C��NaCN��NaHSO3����������DΪ ��D������Һ��ˮ������EΪ
��D������Һ��ˮ������EΪ �����G��SOCl2��Ӧ�����������������ȣ��ж�FΪCH3COCl����֪��RCHO$��_{��NaCN}^{��NaHSO_{3}}$
�����G��SOCl2��Ӧ�����������������ȣ��ж�FΪCH3COCl����֪��RCHO$��_{��NaCN}^{��NaHSO_{3}}$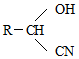 ��RCN$\stackrel{H_{2}O/H+}{��}$RCOOH��RCOOH$\stackrel{SOCl}{��}$RCOCl��
��RCN$\stackrel{H_{2}O/H+}{��}$RCOOH��RCOOH$\stackrel{SOCl}{��}$RCOCl��
������Ҵ�Ϊԭ���Ʊ�F�ĺϳ�·��ΪCH3CH2OH$��_{����}^{O_{2}}$CH3CHO$��_{����}^{O_{2}}$CH3COOH$\stackrel{SOCl_{2}}{��}$CH3COCl��
�ʴ�Ϊ��CH3CH2OH$��_{����}^{O_{2}}$CH3CHO$��_{����}^{O_{2}}$CH3COOH$\stackrel{SOCl_{2}}{��}$CH3COCl��
���� ���⿼�����л���ϳ�·�ߵ��������̷����жϣ�ע�ⷴӦ�����ͷ�Ӧ�����жϣ����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʯ��ʯī�ȶ� | B�� | ʯīת��Ϊ���ʯ��Ҫ���� | ||
| C�� | ���ʯȼ�ղ�����ȶ� | D�� | ������ʱ��ʯī���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϩ��Br2�ӳɣ�CH2�TCHCH3+Br2��CH2BrCH2CH2Br | |
| B�� | ������Cl2����ȡ����Ӧ��CH4+Cl2$\stackrel{����}{��}$CH3Cl+HCl | |
| C�� | ���ѻ���C16H34$��_{��}^{����}$C8H16+C8H18 | |
| D�� | ����ˮ�⣺��C6H10O5��n+nH2O$\stackrel{����}{��}$nC6H12O6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
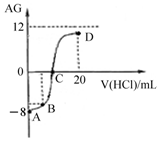 ����AG��ʾ��Һ����ȣ������ʽΪ��AG=lg[$\frac{{c��{H^+}��}}{{c��O{H^-}��}}$]�������£�ʵ��������0.1mol/L��������Һ�ζ�10mL 0.1mol/L MOH��Һ���ζ�������ͼ��ʾ������˵����ȷ���ǣ�������
����AG��ʾ��Һ����ȣ������ʽΪ��AG=lg[$\frac{{c��{H^+}��}}{{c��O{H^-}��}}$]�������£�ʵ��������0.1mol/L��������Һ�ζ�10mL 0.1mol/L MOH��Һ���ζ�������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �õζ����̿�ѡ���̪��Ϊָʾ�� | |
| B�� | ��B������������Һ���Ϊ5 mL��������Һ�У�c��M+��+2c��H+��=c��MOH��+2c��OH-�� | |
| C�� | �ζ������д�A�㵽D����Һ��ˮ�ĵ���̶ȣ�A��B��C��D | |
| D�� | C��ʱ����������Һ���������10 mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������NaOH��Һ��Na+��AlO2-��OH-��SO42- | |
| B�� | ���������ˮ��NH4+��Al3+��OH-��SO42- | |
| C�� | ͨ�����Cl2��Fe2+��Na+��Cl-��SO42- | |
| D�� | ͨ�����SO2��Fe2+��H+��SO32-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����Ҵ��Ļ�ԭ���Լ�Cr3+��Cr2O72-����ɫ����������ƺ�ݳ� | |
| B�� | �ߴ�����Խ�̫����ֱ��ת��Ϊ���� | |
| C�� | ��ѧҩƷ�Ż𣬶�Ҫ������ˮ����ĭ�������� | |
| D�� | ��ҵ��Cl2����ʯ���鷴Ӧ�Ʊ�Ư�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
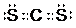 ���÷���Ϊ�Ǽ��ԣ�ѡ����ԡ������Ǽ��ԡ������ӣ�
���÷���Ϊ�Ǽ��ԣ�ѡ����ԡ������Ǽ��ԡ������ӣ��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com