
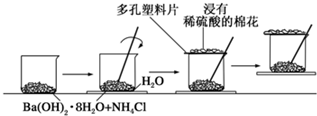
��һ��С�ձ������20g Ba(OH)2��8H2O��ĩ����С�ձ����������ѵ���3��4��ˮ�IJ���Ƭ�ϣ�Ȼ�����10g NH4Cl���壬���ò�����Ѹ�ٽ��裮
(1)ʵ���в�������������________��
(2)д���йط�Ӧ�ķ���ʽ��________���÷�Ӧ��________��Ӧ��
(3)ʵ���й۲쵽��������________��________�ͷ�Ӧ�����ɺ�״����Ӧ�����ʺ�״��ԭ����________��
(4)ͨ��________����˵���÷�ӦΪ________�ȷ�Ӧ���������ڷ�Ӧ���������________���������������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com