


 £¬·“Ó¦¢Ūµē½āČŪČŚµÄNaClæÉŅ±Į¶½šŹōÄĘ£¬
£¬·“Ó¦¢Ūµē½āČŪČŚµÄNaClæÉŅ±Į¶½šŹōÄĘ£¬ £»Ņ±Į¶ÄĘ£»
£»Ņ±Į¶ÄĘ£»


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ¹ć¶«Ź”½ŅŃōŅ»ÖŠøßŅ»µŚŅ»½×¶Īæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
£Ø8·Ö£©ČēĶ¼ĖłŹ¾ŹĒ֊ѧ»ÆѧŹµŃéÖŠ³£¼ūµÄ×°ÖĆ£¬ĖüÓŠ¶ąÖÖÓĆĶ¾”£
£Ø1£©ČōĘæ֊װӊXČÜŅŗ£¬½«COŗĶCO2µÄ»ģŗĻĘųĢåÓÉa¹ÜæŚĶØČė£¬ ÓĆŅŌ³żČ„CO2£¬ŌņXĪŖ________”£
| A£®H2SO4 | B£®NaOH | C£®NaCl | D£®HCl |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŌĘÄĻ»įŌóĻŲÜįĶśøßÖŠøßŅ»ÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
27. ČēĶ¼ĖłŹ¾ŹĒ֊ѧ»ÆѧŹµŃéÖŠ³£¼ūµÄ×°ÖĆ£¬ĖüÓŠ¶ąÖÖÓĆĶ¾”£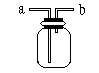
¢ÅČōĘæ֊װӊXČÜŅŗ£¬½«COŗĶCO2µÄ»ģŗĻĘųĢåÓÉa¹ÜæŚĶØČė£¬ÓĆŅŌ³żČ„CO2£¬ŌņXĪŖ________”£
| A£®H2SO4 | B£®NaOH | C£®NaCl | D£®HCl |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015½ģ¹ć¶«Ź”øßŅ»µŚŅ»½×¶Īæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
£Ø8·Ö£©ČēĶ¼ĖłŹ¾ŹĒ֊ѧ»ÆѧŹµŃéÖŠ³£¼ūµÄ×°ÖĆ£¬ĖüÓŠ¶ąÖÖÓĆĶ¾”£

£Ø1£©ČōĘæ֊װӊXČÜŅŗ£¬½«COŗĶCO2µÄ»ģŗĻĘųĢåÓÉa¹ÜæŚĶØČė£¬ ÓĆŅŌ³żČ„CO2£¬ŌņXĪŖ________”£
A£®H2SO4 B£®NaOH C£®NaCl D£®HCl
£Ø2£©ČōÓĆÅÅĖ®·ØŹÕ¼ÆH2£¬ŌņH2ĘųĢåÓ¦“Ó_______£ØĢī±źŗÅ£¬ĻĀĶ¬£©¹ÜæŚµ¼Čė£»ČōÓĆÅÅæÕĘų·ØŹÕ¼ÆCO2£¬ŌņCO2ĘųĢåÓ¦“Ó________ ¹ÜæŚµ¼Čė”£
£Ø3£©Ņ½ŌŗĄļøų²”ČĖŹäŃõŹ±£¬ĶłĶłŌŚŃõĘųøÖĘæÓė²”ČĖŗōĪüĆę¾ßÖ®¼ä°²×°ÓŠĖ®µÄøĆ×°ÖĆ£¬¹Ū²ģĘųÅŻ²śÉśµÄĒéæö£¬ŅŌ±ćµ÷½Ś¹©ŃõĖŁĀŹ£¬“ĖŹ±ŃõĘųÓ¦“Ó ¹ÜæŚµ¼Čė”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģŌĘÄĻ»įŌóĻŲÜįĶśøßÖŠøßŅ»ÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
27. ČēĶ¼ĖłŹ¾ŹĒ֊ѧ»ÆѧŹµŃéÖŠ³£¼ūµÄ×°ÖĆ£¬ĖüÓŠ¶ąÖÖÓĆĶ¾”£
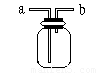
¢ÅČōĘæ֊װӊXČÜŅŗ£¬½«COŗĶCO2µÄ»ģŗĻĘųĢåÓÉa¹ÜæŚĶØČė£¬ÓĆŅŌ³żČ„CO2£¬ŌņXĪŖ________”£
A.H2SO4 B.NaOH C.NaCl D.HCl
¢ĘČōÓĆÅÅĖ®·ØŹÕ¼ÆH2£¬ŌņH2ĘųĢåÓ¦“Ó_______£ØĢī±źŗÅ£¬ĻĀĶ¬£©¹ÜæŚµ¼Čė£»ČōÓĆÅÅæÕĘų·ØŹÕ¼ÆCO2£¬ŌņCO2ĘųĢåÓ¦“Ó ________ ¹ÜæŚµ¼Čė”£
¢ĒŅ½ŌŗĄļøų²”ČĖŹäŃõŹ±£¬ĶłĶłŌŚŃõĘųøÖĘæÓė²”ČĖŗōĪüĆę¾ßÖ®¼ä°²×°ÓŠĖ®µÄøĆ×°ÖĆ£¬¹Ū²ģĘųÅŻ²śÉśµÄĒéæö£¬ŅŌ±ćµ÷½Ś¹©ŃõĖŁĀŹ£¬“ĖŹ±ŃõĘųÓ¦“Ó ¹ÜæŚµ¼Čė”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com