
| W | X | Y |
| Z |
 ��
��| ��� | �����Ʋ� | ��ѧ����ʽ |
| ʾ�� | ������ | H2ZO3+4HI�TZ��+2I2+1H2O |
| �� | ���� | H2SeO3+2NaOH=Na2SeO3+2H2O |
| �� | ��ԭ�� | H2SeO3+Cl2+H2O=H2SeO4+2HCl |
���� ��1������Ԫ�ص�������۵�����������O��F���⣩��Ԫ�������ڱ���λ����������������������ɣ�
��2��ԭ�ӽṹʾ��ͼ����ԭ�Ӻ��Լ�������Ӳ���ɣ�ԭ�Ӻ��ڱ�ʾ���˵���������Ӳ�Ҫע��ÿ�������2n2��������㲻����8��������㲻����18����
��3����������Ϊ�������������������Ӧ�������κ�ˮ��
��4������Ԫ�������ɿ�֪��ͬ����Ԫ�ؾ������Ƶ����ʣ�
��� �⣺��1������������Ԫ��X�����������+5�ۣ�֪XΪ��������Ԫ�أ���WΪ��������Ԫ�أ���Y��ZΪ��������Ԫ�أ��־�Y���ʿ��ڿ�����ȼ�գ�֪Y������������֪Z������X���ף�W�ǹ裬����X��λ��Ϊ�������ڢ�A�壮
�ʴ�Ϊ���������ڢ�A�壻
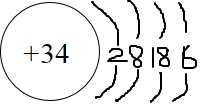
��2����Z������34��Ԫ�أ���Z��ԭ�ӽṹʾ��ͼ��ͼ��ʾ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3����W�����������Ϊ�������裬�����ռ���Һ��Ӧ���ɹ�������ˮ��
�ʴ�Ϊ��SiO2+2NaOH=Na2SiO3+H2O��
��4����Z��������Sͬ���壬���H2ZO3��������H2SO3���ƣ�����Ʋ�����˾��������Ի������ԡ���ԭ�ԣ��������ԣ����������кͷ�Ӧ����������������Ӧ�������仹ԭ�ԣ�
�ʴ�Ϊ�����ԣ�H2SeO3+2NaOH=Na2SeO3+2H2O����ԭ�ԣ�H2SeO3+Cl2+H2O=H2SeO4+2HCl��
���� ������Ҫ������Ԫ���ƶϼ�Ԫ�������ɵ�֪ʶ��������ǿ��ע�ؿ���ѧ���Ի���֪ʶ������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 10mL��������Ϊ98%��H2SO4����10mLˮϡ�ͺ�H2SO4��������������49% | |
| B�� | ������99%�����̲��ڴ��У����屻��Ϊ������Ԫ�ء� | |
| C�� | �ڱ���£���22.4L��������1Lˮ�У��õ�1mol/L�İ�ˮ | |
| D�� | �����ȷݲ����͵��ռ���Һ�зֱ����һ������Na2O2��Na2O��ʹ��Һ��ǡ�ñ��ͣ�������Na2O2��Na2O�����ʵ���֮�ȵ���1��1�������¶Ȳ��䣩 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
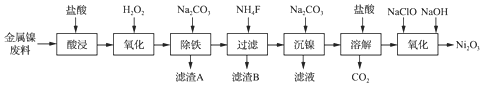
| �������� | Fe��OH��3 | Fe��OH��2 | Ni��OH��2 |
| ��ʼ������pH | 1.1 | 6.5 | 7.1 |
| ������ȫ��pH | 3.2 | 9.7 | 9.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H+��NO3-��Fe2+��Na+ | B�� | Ag+��K+��I-��Cl- | ||
| C�� | K+��Ba2+��OH-��SO42- | D�� | K+��Cu2+��Br-��OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 6.4gO2��O3�Ļ�����壬����ԭ����Ϊ0.4NA | |
| B�� | ���ӱ�״���£�11.2LCCl4�к�������Ϊ0.5NA | |
| C�� | 1mol���ᣨ���ӻӷ���ʧ����������C2H518OH��Ũ���������¼��ȣ���ַ�Ӧ������NA��CH3CO18OC2H5 | |
| D�� | ���³�ѹ�£�7.8gNa2O2��������Ϊ0.4NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ?
? =CH2��g��+H2��g��
=CH2��g��+H2��g��| ʱ��/min | 0 | 10 | 20 | 30 | 40 |
| n���ұ���/mol | 0.40 | 0.30 | 0.24 | n2 | n3 |
| n������ϩ��/mol | 0.00 | 0.10 | n1 | 0.20 | 0.20 |
| A�� | ���������������䣬�������г��벻���뷴Ӧ��ˮ������Ϊϡ�ͼ������ұ���ת���ʴ���50.0% | |
| B�� | ��Ӧ��ǰ20���ӵ�ƽ������Ϊv��H2��=0.008molmol/�� L•min�� | |
| C�� | ����������ƽ��Ħ���������ٱ仯����˵����Ӧ�Ѵﵽƽ��״̬ | |
| D�� | ��ͬ�¶��£���ʼʱ�������г���0.10mol�ұ���0.10mol����ϩ��0.30molH2���ﵽƽ��ǰv����v�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | |||||||
| �� | �� | �� | �� | �� | �� | |||
| �� | �� | �� |
 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
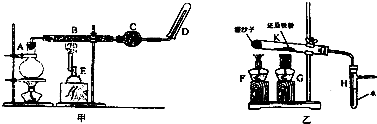
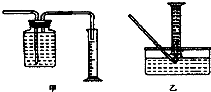
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com