
�����ֶ�����Ԫ�أ����ǵĽṹ�����ʵ���Ϣ���±�������
| Ԫ�� | �ṹ�����ʵ���Ϣ |
| A | �Ƕ�������(��ϡ��������)ԭ�Ӱ뾶����Ԫ�أ���Ԫ�ص�ij�ֺϽ���ԭ�ӷ�Ӧ�ѵĵ��ȼ� |
| B | B��Aͬ���ڣ�������������ˮ��������� |
| C | Ԫ�ص���̬�⻯�K������ˮ������������� |
| D | �Ǻ�ˮ�г��⡢��Ԫ���⺬������Ԫ�أ��䵥�ʻ���Ҳ������ˮ���������г��õ�����ɱ���� |
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ԫ��A��B��C��D��E��ԭ��������������AԪ�������ӵ�ԭ�Ӻ���û�е��ӣ�B�ǿ����к�������Ԫ�أ�CԪ��ԭ������������������Ӳ�����������C��D���γ����ֳ��������ӻ������ҵ�ϳ��õ��C��E�Ļ��������Ʊ�E���ʡ�
��1������DԪ�ص�ԭ�ӽṹʾ��ͼ ��C��D��E�ļ����Ӱ뾶��С�����˳�� �������ӷ��ű�ʾ����
��2��д��Ԫ��E�����ڱ��е�λ�� ��
��3����ҵ�ϳ���A��B�ĵ��ʺϳ�һ�ֳ������壬���������ij��÷����� ��
��4��D2C2��H2O��Ӧ�Ļ�ѧ����ʽ�� ��D2C2��CuSO4��Һ��Ӧ�������� ��
��5����Ԫ��A��B��C��ԭ�Ӹ�����4��2��3�γɵĻ����д������뷽��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������Ԫ��A��B��C��D��E��F��ԭ�����������������ǵ�ԭ
�Ӻ�����Ӳ���֮��Ϊ13��B�Ļ���������࣬��Ŀ�Ӵ�C��D�ǿ����к�����������Ԫ�أ�D��E����Ԫ�صĵ��ʷ�Ӧ�����������ֲ�ͬ�����ӻ����FΪͬ���ڰ뾶��С��Ԫ�ء��Իش��������⣺
(1)д��D��E��1��1��ԭ�Ӹ������γɵĻ�����ĵ���ʽ�� ��F��ԭ�ӽṹʾ��ͼΪ�� ��
(2)B��D�γɵĻ�����BD2�д��ڵĻ�ѧ��Ϊ ��(����ӡ����ۡ�����ͬ)��A��C��F����Ԫ���γɵĻ�����CA4FΪ �����
(3)������ס�����A��B��D��E�е����ֻ�������ɣ��Ҽס��ҵ�ˮ��Һ���ʼ��ԡ���ס��ҷ�Ӧ�����ӷ���ʽΪ�� ��
(4)A��C��D��E��ԭ�Ӱ뾶�ɴ�С��˳���� (��Ԫ�ط��ű�ʾ)��
(5)Ԫ��B��F�ķǽ�����ǿ����B�ķǽ����� ��F(�ǿ��������)�����û�ѧ����ʽ֤���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ԫ��A��B��C��Dԭ����������������CԪ������������Ӧ��ˮ�����ܵ������������ȵ����������ӡ�A��Cλ��ͬһ���壬AΪ�ǽ���Ԫ�أ�B�������������Ǵ�����������3����B��C������������֮����D��������������ȡ�E�����������г�������������Ʒ�ڳ�ʪ�������ױ���ʴ������ش��������⣺
(1)C������������Ӧˮ����Ļ�ѧʽΪ ��
���к��еĻ�ѧ������Ϊ ��
(2)������A��B��C��D����Ԫ���е��������ij���Σ�ˮ��Һ�Լ��ԣ��Ǽ�������������Ҫ�ɷ֡���������Һ����KI������Һ�У���Һ��Ϊ��ɫ����Ӧ�����ӷ���ʽΪ ��
(3)EԪ����DԪ�ؿ��γ�ED2��ED3���ֻ��������˵����ȷ���� (�����)��
�ٱ���ED2��Һʱ��������Һ�м�������E����
��ED2ֻ��ͨ���û���Ӧ���ɣ�ED3ֻ��ͨ�����Ϸ�Ӧ����
��ͭƬ��̼����ED3��Һ���ԭ��أ�������ͭƬ�ص�������̼��
������۵⻯����Һ�ͱ�����Һ�зֱ�μӼ���ED3��Ũ��Һ��ԭ��ɫ��Һ�������ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��A��B��C��D��E���ֶ�����Ԫ��,��֪���ڵ�A��B��C��D����Ԫ��ԭ�Ӻ����56������,�����ڱ��е�λ����ͼ��ʾ��E�ĵ��ʿ����ᷴӦ,1 mol E����������������,�ڱ�״�����ܲ���33.6 L H2;E����������A�������Ӻ�����Ӳ�ṹ��ȫ��ͬ��
�ش���������: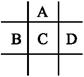
(1)A��E�γɵĻ�����Ļ�ѧʽ������������
(2)B����������ﻯѧʽΪ��������,C��Ԫ������Ϊ��������,D�ĵ�����ˮ��Ӧ�ķ���ʽΪ��____________________________
(3)��D��E�γɵĻ������ˮ��Һ�е����ռ���Һֱ������,�۲쵽��������
_____________________________________________________
�йط�Ӧ�����ӷ���ʽΪ___________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���塣B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1��18���еĵ�7��Ԫ�ء�D��ԭ��������EС5��D��B���γ����ӻ�����侧���ṹ��ͼ��ʾ��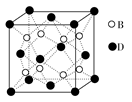
��ش�
(1)AԪ�ص������� ��
(2)B��Ԫ�ط����� ��C��Ԫ�ط����� ��B��A�γɵĻ������C��A�γɵĻ�����е�ߣ���ԭ���� ��
(3)E��Ԫ�����ڱ��е� ���ڣ��� ���Ԫ�أ���Ԫ�������� �����ģ�2�����ӵĵ����Ų�ʽΪ ��
(4)��ͼ�п��Կ�����D��B�γɵ����ӻ�����Ļ�ѧʽΪ �������ӻ����ᄃ����ܶ�Ϊag��cm��3����������� (ֻҪ���г���ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
| Ԫ�ر�� | Ԫ�ص����ʻ�ԭ�ӽṹ |
| X | �����������Ǵ�����������3�� |
| Y | �����µ�����˫ԭ�ӷ��ӣ����⻯���ˮ��Һ�Լ��� |
| Z | ��������Ԫ�صļ������а뾶��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±��г�ǰ20��Ԫ���е�ijЩԪ�����ʵ�һЩ���ݣ�
| Ԫ�� ���� | A | B | C | D | E | F | G | H | I | J |
| ԭ�Ӱ뾶 ��10��10m�� | 1.02 | 2.27 | 0.74 | 1.43 | 0.77 | 1.10 | 0.99 | 1.86 | 0.75 | 1.17 |
| ���̬ | ��6 | ��1 | �� | ��3 | ��4 | ��5 | ��7 | ��1 | ��5 | ��4 |
| ��ͼ�̬ | ��2 | �� | ��2 | �� | ��4 | ��3 | ��1 | �� | ��3 | ��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
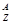 X��ʾԭ�ӣ�
X��ʾԭ�ӣ�
(1)����ԭ�ӵ�������N��______��
(2)AXn������x�����ӣ���������ӵ�������N��______��
(3)AXn������x�����ӣ���������ӵ�������N��______��
(4)12C16O2�����������N��________��
(5)A2��ԭ�Ӻ�����x�����ӣ���������Ϊm����n g A2���������ӵ����ʵ���Ϊ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com