
��4�ֻ����Һ���ֱ��ɵ����0.1 mol·L��1��2����Һ��϶��ɣ���CH3COONa��HCl����CH3COONa��NaOH����CH3COONa��NaCl����CH3COONa��NaHCO3
���и���������ȷ����(����)
A��pH����>��>��>��
B��c(CH3COO��)����>��>��>��
C����Һ��c(H��)����>��>��>��
D��c(CH3COOH)����>��>��>��
��������Ϻ�ٷ�Ӧ����CH3COOH��NaCl�������Ϻ������Ӧ������ܱȽϣ�����NaHCO3ˮ��̶ȴ���CH3COONa�����Ԣܵ�pH���ڢۣ���pH��С˳��Ϊ����>��>��>�٣���c(H��)��С˳��Ϊ����>��>��>�ڣ���A��C������ڢ���CH3COO����ˮ����ܵ����ƣ�����OH����CH3COO����ˮ�����Ƴ̶ȱȢ�ǿ�����Կɵ�c(CH3COO��)��С˳��Ϊ����>��>��>�٣���c(CH3COOH)��С˳�������෴Ϊ����>��>��>�ڣ���B����ȷ��D�����
�𰸡�B


 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���ǣ� ��
A��ˮ�����ӻ�����KWֻ���¶��йأ�������ᡢ���һ����Ӱ��ˮ�ĵ���̶�
B��Ksp���������ܵ���ʵ����ʺ��¶��йأ�������Һ��������ӵ�Ũ���й�
C�������£���0.10 mol��L��1��NH3��H2O��Һ�м�������NH4Cl���壬��ʹ��Һ��pH��С��c(NH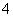 )/c(NH3��H2O) ��ֵ����
)/c(NH3��H2O) ��ֵ����
D�������£�CH3COOH��Ka��1.7��10��5��NH3��H2O��Kb��1.7��10��5��CH3COOH��Һ�е�c(H��) ��NH3��H2O�е�c(OH��) ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
W��X��Y��Z��ԭ���������������ͬһ������Ԫ�أ�W��X�ǽ���Ԫ�أ�Y��Z�Ƿǽ���Ԫ�ء�
(1)W��X���Ե�����������Ӧ��ˮ������Է�Ӧ�����κ�ˮ���÷�Ӧ�����ӷ���ʽΪ_______________________________��
(2)W��Y���γɻ�����W2Y���û�����Ļ�ѧʽΪ________��
(3)�Ƚ�Y��Z��̬�⻯����ȶ��ԣ�________(�û�ѧʽ��ʾ)��
(4)W��X��Y��Z����Ԫ�ؼ����ӵİ뾶�ɴ�С��˳����________(�û�ѧʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Դ��������������ͷ�չ����Ҫ���ʻ�����������Դ�ĺ������ú�����Դ�Ŀ����ǵ�������������ٵ��Ͼ����⡣�ش��������⣺
(1)�ҹ���������������úΪ��Ҫȼ�ϵĹ��ң����й���ú��ȼ�ϵ��۵���ȷ����(����)
A��ú����Ҫ�Ļ���ԭ�ϣ���ú��ȼ�ϼ�ȼ�յ�̫��ϧ��Ӧ���ۺ�����
B��ú�Ƿ��ȺܸߵĹ���ȼ�ϣ��ҹ�ú̿��Դ��Լ��У����ɳɱ��ͣ���ú��ȼ�Ϻ���
C��úȼ��ʱ������������������̳����Ի�����Ⱦ����
D��ͨ���ྻú��������ú��������Һ���Լ�����������������ȼú��Ⱦ���������úȼ�յ���������
(2)�ڿ�����ѧ�ҽ�ͭ��������ۻ��Ƴɶ��������������̫�ջ����ʹ�õ�ú��ȼ��������������������������________________________________________________________��
(3)�Ҵ���δ����ȼ������ѡ������Һ��ȼ�ϣ�����������ɫֲ��Ľո���ȡ��1.0 g�Ҵ���ȫȼ������Һ̬ˮ�ų�29.72 kJ��������ʾ�Ҵ�ȼ�յ��Ȼ�ѧ����ʽΪ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£�ij��Һ����ˮ���������c(OH��)��10��12mol·L��1�������Һ�����ʲ�������(����)
A��HCl B��NaOH
C��NH4NO3 D��H2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10 mL 0.1 mol·L��1 NH4Al(SO4)2��Һ�У��μӵ�Ũ��Ba(OH)2��Һx mL������������ȷ����(����)
A��x��10ʱ����Һ����NH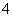 ��Al3����SO
��Al3����SO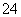 ����c(NH
����c(NH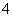 )>c(Al3��)
)>c(Al3��)
B��x��10ʱ����Һ����NH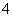 ��AlO
��AlO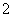 ��SO
��SO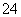 ����c(NH
����c(NH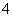 )>c(SO
)>c(SO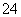 )
)
C��x��30ʱ����Һ����Ba2����AlO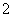 ��OH������c(OH��)<c(AlO
��OH������c(OH��)<c(AlO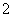 )
)
D��x��30ʱ����Һ����Ba2����Al3����OH������c(OH��)��c(Ba2��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���20 mL����������Һ����μ���0.2 mol·L��1��CH3COOH��Һ���ζ�������ͼ��ʾ��

(1)������������Һ�����ʵ���Ũ��Ϊ______________��
(2)��b�㣬c(Na��)________c(CH3COO��)(�����������������)��
(3)����������Һ��CH3COOH��Һǡ����ȫ��Ӧ�ĵ�λ�����ߵ�________(��ѡ��ı��)��
A��a�� B��b��
C��d�� D��a��b���ij��
E��b��d���ij��
(4)��d�㣬��Һ���������ӵ�Ũ���ɴ�С��˳��Ϊ
_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���������(����)
A���Ҵ������ᡢ�����������ܷ���ȡ����Ӧ
B���۱�ϩ�Ǹ߷��ӻ������������
C��������ϩ�����������ϱ�Ĥ����Ʒ���䵥����CH2CH2Cl
D����ȩ��һ������������CH2—O�ķ�Ӧ���ڼӾ۷�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��ͬһ�����ڵ����ֶ�����Ԫ�ء�A��B��C��ԭ�������������1��AԪ�صĵ��ʻ�ѧ���ʺܻ��ã�AԪ�ص�ԭ���ڸ�������ԭ�Ӱ뾶���(ϡ������Ԫ�س���)��BԪ�ص�������2.0 gǡ�ø�100 mL 0.5 mol·L��1��ϡ���ᷴӦ��BԪ�صĵ�����DԪ�صĵ��ʷ�Ӧ���ɻ�����BD2������������ʵ��գ�
(1)Aԭ�ӵĽṹʾ��ͼΪ________��Cԭ�ӵĵ���ʽΪ_____��
(2)�õ���ʽ��ʾBD2���γɹ���_____________________��
(3)����C���������м���D���⻯���ˮ��Һ����Ӧ�����ӷ���ʽΪ__________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com