
���ĵð������������ಡ��ҩ�����������һ�ֺϳ�·��(���巴Ӧ�����Ͳ����Լ���)��
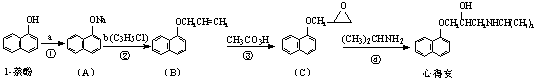
�ش��������⣺
��1���Լ�b�Ľṹ��ʽΪ_______________��b�й����ŵ�������__________________��
��2���۵ķ�Ӧ������________________��
��3���ĵð��ķ���ʽΪ_________________��
��4���Լ�b���ɱ��龭������Ӧ�ϳɣ�
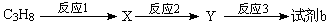 ��Ӧ1���Լ�������Ϊ________________����Ӧ3�ķ�Ӧ������_________________��
��Ӧ1���Լ�������Ϊ________________����Ӧ3�ķ�Ӧ������_________________��
X�ĺ˴Ź���������ʾ������к���2����ԭ�ӣ���Ӧ2�Ļ�ѧ����ʽΪ��
_________________________________________________����һ��ȡ��������X�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ���_____________________________________��
��5�����㻯����D��1-���ӵ�ͬ���칹�壬������������������ţ��ܷ���������Ӧ��D�ܱ�KMnO4������Һ������E(C2H4O2)�ͷ��㻯����F(C8H6O4)��E��F��̼��������Һ��Ӧ���ܷų�CO2���壬F�����ϵ�һ��������ֻ��һ�֡�
D�Ľṹ��ʽΪ__________________________����F����һ��������Ļ�ѧ����ʽΪ��
__________________________________________________________________________�����㻯����F��������________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ��R��X��T��Z��Q��Ԫ�����ڱ��е����λ�����±���ʾ,����R�����ڰ�����H2���һ��ϲ�������ը���������ж���ȷ����
��(����)
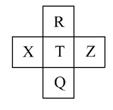
A.�ǽ�����:Z<T<X
B.R��Q�ĵ��������26
C.��̬�⻯���ȶ���:R<T<Q
D.����������ˮ���������:T>Q
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A����Է���������ͬ�ļ��ֻ��������Ϊͬ���칹��
B��ij�л���ȼ��ֻ����CO2��H2O���Ҷ������ʵ�����ͬ������л�������ΪCnH2n
C������ͬһͨʽ���л���˴�һ����ͬϵ��
D�����Ԫ�ص�����������ͬ������Է�������Ҳ��ͬ�IJ�ͬ�����һ����Ϊͬ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��25��ʱ����⺬��0.02 mol CuSO4��0.04 mol NaCl�Ļ����Һ200mL��������
����672mL����״���£�����ʱ�����������Һ����仯���������Һ��PHΪ�� ��
A�� 1 B��2 C��6 D��7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�ԭ�ӽṹ��Ԫ�������ɵ���������ȷ����
A��ͬ��Ԫ�ص�ԭ�Ӿ�����ͬ����������������
B��ͬ����Ԫ�صļ������ӻ�ԭ��Խǿ��ˮ��̶�Խ��
C��ͬ���ڽ���Ԫ�صĻ��ϼ�Խ�ߣ���ԭ��ʧ��������Խǿ
D�������ڵڢ�A���A��Ԫ�ص�ԭ�Ӽ乹�ɵķ��ӣ�������ԭ�������8���ӽṹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й����л����˵��������ǡ�(����)
A.CCl4����CH4�Ƶ�,����ȡ��ˮ�еĵ�
B.�����ʵ������Ҵ�����ϩ��ȫȼ��ʱ�����������������
C.�Ҵ�������������������ñ���Na2CO3��Һ����
D.������ʹKMnO4��Һ��ɫ,˵�������ܷ���������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������һ����Ҫ�Ļ�����Ʒ,�����������������Ϊһ�����ҹ�ҵ��չˮƽ��һ�ֱ�־��Ŀǰ����Ҫ���������ǡ��Ӵ�����,�йؽӴ�������Ӧ2SO2+O2 2SO3��˵������ȷ���ǡ�(����)
2SO3��˵������ȷ���ǡ�(����)
A.�÷�ӦΪ���淴Ӧ,����һ�������¶������������������ȫ��ת��Ϊ��������
B.�ﵽƽ���,��Ӧ��ֹͣ��,�ʴ�ʱ�����淴Ӧ��������Ҿ�Ϊ��
C.һ��������,��ij�ܱ������м���2 mol SO2��1 mol O2,��ӷ�Ӧ��ʼ���ﵽƽ��Ĺ�����,����Ӧ���ʲ��ϼ�С,�淴Ӧ���ʲ�������,ijһʱ��,�����淴Ӧ�������
D.������������Ӧ������������ʱ,Ҫͬʱ���Ƿ�Ӧ���ܴﵽ���Ⱥͻ�ѧ��Ӧ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������,��20 mL 0.2 mol��L-1H2A��Һ�еμ�0.2 mol��L-1NaOH��Һ���й��������ʵ����仯��ͼ(���Т����H2A,�����HA-,�����A2-)������ͼʾ�ж�����˵����ȷ����(����)
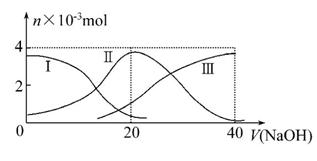
A.H2A�ĵ��뷽��ʽΪH2A A2-+2H+
A2-+2H+
B.��NaHA��Һ��:c(H+)=c(A2-)+c(OH-)-c(H2A)
C.��V(NaOH)=20 mLʱ,��Һ������Ũ�ȴ�С��ϵ:c(Na+)>c(HA-)>c(OH-)>c(A2-)>c(H+)
D.��NaHA��Һ����ˮ�Ĺ�����,pH��������Ҳ���ܼ�С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йؾ����˵��
�����ݹ������ӵĶѻ���ʽ�ɽ������Ϊ�������塢���Ӿ��塢���Ӿ��塢ԭ�Ӿ��壻����ԭ��ֱ�ӹ��ɵľ������ԭ�Ӿ��壻�۷��Ӿ���Ķѻ�����ȡ�����ܶѻ�����SiF4��NaF��MgF2 ���־�����۵��������ߣ��ݽ�����ֻ�����ڽ��������У������Ӽ�ֻ���������Ӿ����У���H2O�����ʷdz��ȶ���ԭ�����ڷ���֮������������SO2��SiO2�������ۻ�ʱ�ƻ�������������ͬ������һ����ȷ����
A��3�� B��4�� C��5�� D��6��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com