
| A��ԭ�����Һ��ֻ����Na+��Fe3+��SO42-�������ܴ���K+��CO32- |
| B����ʵ������ƶ�ԭ�����Һ���Ƿ���SO42- |
| C����ʵ��١��ڿ��ж�ԭ�����Һ���Ƿ���Fe3+ |
| D����ʵ��ۿ��ж�ԭ�����Һ�д���Fe2+ |


 �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
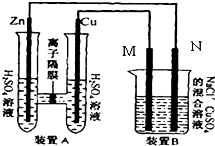 ��ͼװ��B����Ũ�Ⱦ�Ϊ0.1mol/L��NaCl��CuSO4�����Һ����Һ���Ϊ500ml��M��N��Ϊʯī�缫����װ��A��Zn����������6.5gʱ��N����������
��ͼװ��B����Ũ�Ⱦ�Ϊ0.1mol/L��NaCl��CuSO4�����Һ����Һ���Ϊ500ml��M��N��Ϊʯī�缫����װ��A��Zn����������6.5gʱ��N�����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����NH3ͨ���ȵ�CuSO4��Һ����ʹCu2+��ԭ��Cu |
| B�������£�������ˮ���ܴ�������C1-��Fe3+��NO3-��Na+��SO32- |
| C��Ũ�Ⱥ������ͬ��NaOH��CH3COOH���ϣ���Һ��c��Na+����c��CH3COO-�� |
| D���ŵ練ӦΪH2+2NiO��OH��=2Ni��OH��2�ĵ�أ����ʱ��������NiO��OH������ԭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��pH=4�������pH=10�İ�ˮ |
| B��pH=4�Ĵ�����Һ��pH=10������������Һ |
| C��0.1mol?L-1�������0.1mol?L-1������������Һ |
| D��0.1mol?L-1�������0.1mol?L-1������������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����״���£�11.2 L�����к���0.5 NAԭ�� |
| B�����³�ѹ�£�46g NO2��N2O4��������к���ԭ������Ϊ3NA |
| C����״���£�0.1 mol Cl2�μӷ�Ӧ��ת�Ƶĵ�����Ŀһ��Ϊ0.2 NA |
| D��1 mol Na������O2��Ӧ������Na2O��Na2O2�Ļ���ת�Ƶ�������NA�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com