
����Ŀ����1mol N2O4��������ݻ�Ϊ10L���ܱ������У��ش��������⣺

��1��100 ��ʱ����ϵ�и�����Ũ����ʱ��仯����ͼ��ʾ����0~60 sʱ�Σ���Ӧ����v(N2O4)Ϊ________________����ӦN2O4(g)![]() 2NO2(g)��ƽ�ⳣ��KΪ___________��
2NO2(g)��ƽ�ⳣ��KΪ___________��
��2������Ӧ�ﵽƽ��ʱ�����ڷ�ӦN2O4(g)![]() 2NO2(g)���ı�ijһ����������˵���У�һ����˵����ѧƽ��������Ӧ�����ƶ�����____________��
2NO2(g)���ı�ijһ����������˵���У�һ����˵����ѧƽ��������Ӧ�����ƶ�����____________��
��������ɫ����
��N2O4�������������
�ۺ��º�ѹ����He
�ܵ�λʱ��������N2O4��NO2�����ʵ���֮�ȵ���1��2
��3������ʼʱ����1molNO2���壬��������ƽ��2NO2(g)![]() N2O4(g)�����NO2��ת����Ϊa%�����¶ȡ��������ʱ���ٳ���1molNO2���壬���´ﵽƽ��ʱ�����NO2��ת����Ϊb%����a______b������>������<������=�����������º�ѹʱ������1molNO2���壬��Ӧ2NO2(g)
N2O4(g)�����NO2��ת����Ϊa%�����¶ȡ��������ʱ���ٳ���1molNO2���壬���´ﵽƽ��ʱ�����NO2��ת����Ϊb%����a______b������>������<������=�����������º�ѹʱ������1molNO2���壬��Ӧ2NO2(g)![]() N2O4(g)�ﵽƽ�������������ͨ��һ������NO2���壬���´ﵽƽ��ʱ��NO2���������_________����������������������������С������
N2O4(g)�ﵽƽ�������������ͨ��һ������NO2���壬���´ﵽƽ��ʱ��NO2���������_________����������������������������С������
��4������ͼa��ʾ����ͨ�IJ���ƿ�г���NO2���壬��������ƽ��2NO2(g)![]() N2O4(g)����֪Fe3+��H2O2�ķֽ���д����ã�����ͼb��c�е���Ϣ���Ʋ�Aƿ��������ɫ��Bƿ�е�____________��������������dz������
N2O4(g)����֪Fe3+��H2O2�ķֽ���д����ã�����ͼb��c�е���Ϣ���Ʋ�Aƿ��������ɫ��Bƿ�е�____________��������������dz������

���𰸡�0.001mol/(L��s) 0.36 �� < ���� dz
��������
��1��������ͼ�����ݿ�֪����0~60 sʱ�Σ�c(N2O4)����0.100 mol��L��1��0.040 mol��L��1����60 s��0.001 mol��L��1��S-1����Ӧ������60s��Ӧ���Ũ�ȱ��ֲ��䣬˵����Ӧ�ﵽƽ��״̬����ӦN2O4(g)![]() 2NO2(g)��ƽ�ⳣ��K=
2NO2(g)��ƽ�ⳣ��K=![]() =
=![]() =0.36�� �ʴ�Ϊ��0.001mol/(L��s)��0.36��
=0.36�� �ʴ�Ϊ��0.001mol/(L��s)��0.36��
��2��������С���ʹc(NO2)����������ɫ�����ƽ���ʱ���淴Ӧ�����ƶ���
���ں��º���ʱ������NO2��ʹƽ�������ƶ���N2O4������������ӣ���N2O4������������Ӳ���˵��ƽ�������ƶ���
�ۺ��º�ѹ����He���������ƽ��������Ӧ�����ƶ���
�ܵ�λʱ��������N2O4��NO2�����ʵ���֮�ȵ���1��2��˵����ʱ���淴Ӧ������ȣ���Ӧ����ƽ��״̬��
��������������������У��� ���ʴ�Ϊ������
��3��������䣬�ﵽƽ����ٳ���1mol NO2���൱�ڼ�ѹ��ƽ�������ƶ���ת��������b%��a%���ں��º�ѹʱ����Ӧ2NO2(g)![]() N2O4(g)�ﵽƽ������������ڳ���һ������NO2��������ɱ䣬����ƽ���Ч����N2O4������������䣻�ʴ�Ϊ��<�����䣻
N2O4(g)�ﵽƽ������������ڳ���һ������NO2��������ɱ䣬����ƽ���Ч����N2O4������������䣻�ʴ�Ϊ��<�����䣻
��4����ͼb֪H2O2�ֽ���ȣ���ͼa��B�������¶Ƚ�A�����ߣ���ͼc��֪��Ӧ2NO2(g)![]() N2O4(g)����ӦΪ���ȷ�Ӧ�������¶ȣ�ʹƽ��2NO2
N2O4(g)����ӦΪ���ȷ�Ӧ�������¶ȣ�ʹƽ��2NO2![]() N2O4�����ƶ��� NO2��Ũ���������������ɫ�����B��Ũ�Ƚϸߣ��������������ɫ���A��������ɫ��B��dz���ʴ�Ϊ��dz��
N2O4�����ƶ��� NO2��Ũ���������������ɫ�����B��Ũ�Ƚϸߣ��������������ɫ���A��������ɫ��B��dz���ʴ�Ϊ��dz��


 �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ�������ʵ�鷽�����Է���KCl��BaCl2���ֹ�������Իش��������⣺

��1�������ٵ�������_____�������ڵ�������____��
��2���Լ�a��_____(�ѧʽ����ͬ)���Լ�b��____������B��_____��
��3�������Լ�a��������Ӧ�Ļ�ѧ����ʽΪ_______________________�������Լ�b��������Ӧ�Ļ�ѧ����ʽΪ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����ͼ��ʾ��ʵ��װ���о�����X�����ʡ�����X����Ҫ�ɷ������������л�����ˮ��������ش��������⣺
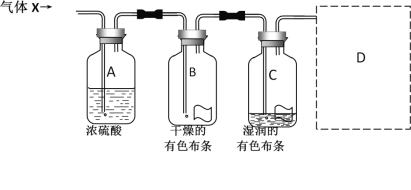
(1)��ʵ�����ҪĿ����__________________________��
(2)��ʵ��Ŀ��ֱ����ص�ʵ��������_________________________��
(3)ͼ����ʾ��ʵ����ƻ����ڲ��㡣�����������ʵ����ʣ���ͼ�е�D����ͼ�����й�ʵ��װ�ú������Լ���_______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ӦC��s����H2O��g��![]() CO��g����H2��g����һ����ɱ�������н��С����������ĸı���仯ѧ��Ӧ���ʼ���û��Ӱ����ǣ�������C���������ڽ����������Сһ�룻�۱�������������䣬���뵪��ʹ������ѹǿ���ܱ���ѹǿ���䣬���뵪��ʹ������������
CO��g����H2��g����һ����ɱ�������н��С����������ĸı���仯ѧ��Ӧ���ʼ���û��Ӱ����ǣ�������C���������ڽ����������Сһ�룻�۱�������������䣬���뵪��ʹ������ѹǿ���ܱ���ѹǿ���䣬���뵪��ʹ������������
A. �ڢ� B. �٢� C. �ۢ� D. �٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪t ��ʱ��0.01 mol/L NaOH��Һ��pH��11��0.1 mol/L��HA��Һ��c(H+)/c(OH-)=109
��ش��������⣺
��1�����¶��£�ˮ�����ӻ�Kw��_____��0.1 mol/L��HA��Һ��ˮ�������c(OH-)=_____��
��2���������£���pH֮��Ϊ14��NaOH��Һ��HA��Һ�������Ϻ�������Һ��_____(��ᡱ������С�)�ԡ�
��3���������£�����ˮϡ��0.01 mol/L HA��Һʱ�����гʼ�С���Ƶ���_____��
A��ˮ�ĵ���̶� B��c(HA)/c(A-)
C����Һ��c(H��)��c(OH��)�ij˻� D����Һ��c(A��)��c(HA)��ֵ
��4�������£�ȡpH��2�������HA��Һ��100 mL�������зֱ����������Zn������Ӧ����������Һ��pH�仯��ͼ��ʾ��
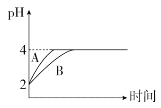
��ͼ�б�ʾHA��ҺpH�仯���ߵ���____(�A����B��)��
���������м���Zn������Ϊm1��HA��Һ�м���Zn������Ϊm2����m1_____m2(�>����<������)��
��5�������£�ȡ0.01 mol/L�������HA��Һ��100 mL���ֱ�μ�0.01 mol/LNaOH��Һ��ǡ����ȫ��Ӧ������NaOH��Һ�����ǰ��____����(�>����<������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������ȡ�����ķ�Ӧ��2H2O2=2H2O+O2�����������£���Ӧ�����������ǣ� ��
ѡ�� | H2O2���������� | �¶� | ���� |
A | 5% | 5�� | MnO2 |
B | 5% | 40�� | MnO2 |
C | 10% | 5�� | �� |
D | 10% | 40�� | MnO2 |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NAΪ����٤��������ֵ������������ȷ����
A. 1 mol Na2O2���������4NA������
B. ��״���£�22.4LCH3OH����NA������
C. �����£�1LpH=1��H2SO4��Һ�У�����0.2NA��H+
D. ��״���£�22.4LN2��O2�Ļ�������к��е�ԭ����Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ʻ���(COS)����������ˮ�ڴ��������µķ�Ӧ���£�
��COS(g)+H2(g)![]() H2S(g)+CO(g) ��H1=-17kJ/mol��
H2S(g)+CO(g) ��H1=-17kJ/mol��
��COS(g)+H2O(g)![]() H2S(g)+CO2(g) ��H2=-35kJ/mol��
H2S(g)+CO2(g) ��H2=-35kJ/mol��
�ش��������⣺
(1)������Ӧ������ѧ�����ƾ�������ԭ���ǣ�________________��
(2)��ӦCO(g)+H2O(g)![]() H2(g)+CO2(g)����H=_______��
H2(g)+CO2(g)����H=_______��
(3)�ʻ���������ˮ����������ϵ��ʼͶ�ϱȲ��䣬����ʻ�����ˮ������Ӧ��ѡ���ԵĹؼ�������______��
(4)�ڳ��д����ĺ�ѹ�ܱ�������ֻ���з�Ӧ��![]() ����ʼ�����n(H2)��n(COS)=m����ͬʱ���ڲ��COSת������m���¶�(T)�Ĺ�ϵ��ͼ��ʾ��
����ʼ�����n(H2)��n(COS)=m����ͬʱ���ڲ��COSת������m���¶�(T)�Ĺ�ϵ��ͼ��ʾ��

��m1______m2(����![]() ������
������![]() ������
������![]() ��
��![]() ��
��
���¶ȸ���T0��COSת���ʼ�С�Ŀ���ԭ��Ϊ��i�и�Ӧ������ii______��iii______��
(5)�ڳ��д����ĺ�ѹ�ܱ������н��з�Ӧ��.COS(g)��H2O(g)Ͷ�ϱȷֱ�Ϊ1��3��1��1����Ӧ��������ʵ�����ͬʱ��COS(g)��ƽ��ת�������¶ȵĹ�ϵ������ͼ��ʾ��
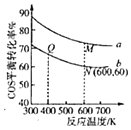
��M���Ӧ��ƽ�ⳣ��______Q��![]() ����
����![]() ������
������![]() ������
������![]() ��
��![]() ��
��
��N���Ӧ��ƽ��������COS(g)���ʵ�������Ϊ______��
��M���Q���Ӧ��ƽ��������������ʵ���֮��Ϊ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪������(H3PO3)�Ľṹ��ͼ ������ǿ��ԭ�Ե����ᣬ���Ա�����������Ϊ���ᡣ
������ǿ��ԭ�Ե����ᣬ���Ա�����������Ϊ���ᡣ
(1)��֪���������PCl3ˮ���������д����Ӧ�����ӷ���ʽ__________.
(2)Na2HPO3��____(����������������ʽ����) ����
(3)�������������ӷ�Ӧʱ�������뻹ԭ�������ʵ���֮��Ϊ______________��
(4)ij�¶��£�0.10molL-1��H3PO3��Һ��pHΪ1.6����c(H+)=2.5��10-2molL-1�����¶���H3PO3�ĵ���ƽ�ⳣ��K=___________________��(H3PO3�ڶ���������Բ��ƣ����������λ��Ч����)
(5)��H3PO3��Һ�еμ�NaOH��Һ�����ԣ�������Һ�У�c(Na+)_____c(H2PO3-)+2c(HPO32-)(��������������������=������ͬ)����NaH2PO3��Һ�У�c(H+)+c(H3PO3)_____c(HPO32-)+c(OH-)
(6)��ijŨ�ȵ��������еμ�NaOH��Һ����pH����Һ�е�H3PO3��H2PO3-��HPO32-�����ʵ�������a(X)��ƽ��ʱij���ֵ�Ũ������������Ũ��֮�͵ı�ֵ)�Ĺ�ϵ��ͼ��ʾ��

�Է�̪Ϊָʾ��������Һ����ɫ��Ϊdz��ɫʱ��������Ҫ��Ӧ�����ӷ���ʽ��_____________.
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com