
| A������Na2O2�����Ƿ����ΪNa2CO3���������м������ᣬ������ɫ��ζ������ |
| B����ȥ�����е�Ca2+��SO42-�����μ������BaCl2��Һ��Na2CO3��Һ�����˺��ټ��������� |
| C����������ˮ������Ƿ���л�ԭ�ԣ���ˮ������Һ�м�������Cu��OH��2������ |
| D��������Һ���Ƿ�һ������CO2-3���μ�ϡ���ᣬ������������ͨ�����ʯ��ˮ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ��˳�� | ʵ������ | ʵ������ |
| �� | A+B | û�������� |
| �� | B+D | ������ų� |
| �� | C+B | ������� |
| �� | A+D | ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���� | B���٢� | C���ڢ� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ӣ�����Ũ��ˮ���� |
| B�����飨��ϩ���������� |
| C���������������ᣩ���ӱ���̼������Һ��Һ |
| D���Ҵ���ˮ������������ʯ��,���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ��Һ��������ͬʱ���ȣ����������ʵ������������٣����������г���δ�ܽ⣬�ڴ˹����л��д̼�����ζ�������ɡ�
��Һ��������ͬʱ���ȣ����������ʵ������������٣����������г���δ�ܽ⣬�ڴ˹����л��д̼�����ζ�������ɡ�A��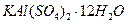 | B��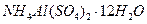 |
C��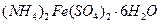 | D��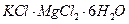 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ���ʣ�������Ϊ���ʣ� | �� | �����Լ� | ||||
| (1) CO2���壨SO2�� | |
| ||||
| (2) CH4���壨CH2=CH2�� | | |||||
| (3) Na2CO3���壨NaHCO3�� | | |||||
| (4) FeCl2��Һ��FeCl3�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ð�ˮϴ�Ӹ����������Թ� |
| B����CCl4����ȡ��ֱ�ӴӺ�������ȡI2 |
| C���������ſ������ռ�NH3������ʪ�����ɫʯ����ֽ���� |
| D������Ȳʱ�μӱ���ʳ��ˮ��Ϊ�˼�����ʯ��ˮ�ķ�Ӧ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com