
����Ŀ���ʻ���(COS)����������ˮ�ڴ��������µķ�Ӧ���£�
��COS(g)+H2(g)![]() H2S(g)+CO(g) ��H1=-17kJ/mol��
H2S(g)+CO(g) ��H1=-17kJ/mol��
��COS(g)+H2O(g)![]() H2S(g)+CO2(g) ��H2=-35kJ/mol��
H2S(g)+CO2(g) ��H2=-35kJ/mol��
�ش��������⣺
(1)������Ӧ������ѧ�����ƾ�������ԭ���ǣ�________________��
(2)��ӦCO(g)+H2O(g)![]() H2(g)+CO2(g)����H=_______��
H2(g)+CO2(g)����H=_______��
(3)�ʻ���������ˮ����������ϵ��ʼͶ�ϱȲ��䣬����ʻ�����ˮ������Ӧ��ѡ���ԵĹؼ�������______��
(4)�ڳ��д����ĺ�ѹ�ܱ�������ֻ���з�Ӧ��![]() ����ʼ�����n(H2)��n(COS)=m����ͬʱ���ڲ��COSת������m���¶�(T)�Ĺ�ϵ��ͼ��ʾ��
����ʼ�����n(H2)��n(COS)=m����ͬʱ���ڲ��COSת������m���¶�(T)�Ĺ�ϵ��ͼ��ʾ��

��m1______m2(����![]() ������
������![]() ������
������![]() ��
��![]() ��
��
���¶ȸ���T0��COSת���ʼ�С�Ŀ���ԭ��Ϊ��i�и�Ӧ������ii______��iii______��
(5)�ڳ��д����ĺ�ѹ�ܱ������н��з�Ӧ��.COS(g)��H2O(g)Ͷ�ϱȷֱ�Ϊ1��3��1��1����Ӧ��������ʵ�����ͬʱ��COS(g)��ƽ��ת�������¶ȵĹ�ϵ������ͼ��ʾ��
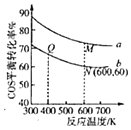
��M���Ӧ��ƽ�ⳣ��______Q��![]() ����
����![]() ������
������![]() ������
������![]() ��
��![]() ��
��
��N���Ӧ��ƽ��������COS(g)���ʵ�������Ϊ______��
��M���Q���Ӧ��ƽ��������������ʵ���֮��Ϊ______��
���𰸡�������Ӧ��Ϊ���Ƚ��ٵķ�Ӧ -18kJ/mol ѡ���Ч�Ĵ��� ![]() ����������� ƽ��������� < 20% 1��1
����������� ƽ��������� < 20% 1��1
��������
(1)�����Ȼ�ѧ����ʽ�����֪��������Ӧ���������ٵķ�Ӧ������Ӧ����������������С���������ѧ����С��
(2) ��COS(g)+H2(g)![]() H2S(g)+CO(g) ��H1=-17kJ/mol��
H2S(g)+CO(g) ��H1=-17kJ/mol��
��COS(g)+H2O(g)![]() H2S(g)+CO2(g) ��H2=-35kJ/mol��
H2S(g)+CO2(g) ��H2=-35kJ/mol��
��˹���ɼ����-��õ���CO(g)+H2O(g)![]() H2(g)+CO2(g)����H��
H2(g)+CO2(g)����H��
(3)����ʻ�����ˮ������Ӧ��ѡ���ԵĹؼ������Ǹ�Ч������
(4)���ڳ��д����ĺ�ѹ�ܱ�������ֻ���з�Ӧ������ʼ�����n(H2)��n(COS)=m��mԽ��˵��������Խ�࣬���ַ�Ӧ������һ�ֻ������һ�ֵ�ת���ʣ�
���¶ȸ���T0��COSת���ʼ�С����Ϊ�¶����ߣ��������Լ�������Ӧ������ƽ��������У�COS��ת���ʼ�С��
(5)�ٷ�Ӧ��ƽ�ⳣ�����¶ȱ仯������ƽ��������У�
��N��COSת����Ϊ60%��COS(g)��H2O(g)Ͷ�ϱ�1��1��������м�����ʽ����N���Ӧ��ƽ��������COS(g)���ʵ���������
��M��COSת����Ϊ60%��COS(g)��H2O(g)Ͷ�ϱ�1��3��N��COSת����Ϊ60%��COS(g)��H2O(g)Ͷ�ϱ�1��1����Ӧǰ���������ʵ���������㡣
(1)��COS(g)+H2(g)![]() H2S(g)+CO(g) ��H1=-17kJ/mol��
H2S(g)+CO(g) ��H1=-17kJ/mol��
��COS(g)+H2O(g)![]() H2S(g)+CO2(g) ��H2=-35kJ/mol��
H2S(g)+CO2(g) ��H2=-35kJ/mol��
������Ӧ�ų����������٣����������Ӧ������ѧ�����ƾ�����
(2)��COS(g)+H2(g)![]() H2S(g)+CO(g) ��H1=-17kJ/mol��
H2S(g)+CO(g) ��H1=-17kJ/mol��
��COS(g)+H2O(g)![]() H2S(g)+CO2(g) ��H2=-35kJ/mol��
H2S(g)+CO2(g) ��H2=-35kJ/mol��
���ݸ�˹���ɼ����![]() ��õ���CO(g)+H2O(g)
��õ���CO(g)+H2O(g)![]() H2(g)+CO2(g)����H=-18kJ/mol��
H2(g)+CO2(g)����H=-18kJ/mol��
(3)�ʻ���������ˮ����������ϵ��ʼͶ�ϱȲ��䣬����ʻ�����ˮ������Ӧ��ѡ���ԵĹؼ������ǣ�ѡ���Ч�Ĵ�����
(4)���ڳ��д����ĺ�ѹ�ܱ�������ֻ���з�ӦCOS(g)+H2(g)![]() H2S(g)+CO(g) ��H1=-17kJ/mol������ʼ�����n(H2)��n(COS)=m��mԽ����������Խ�࣬�����COS��ת���ʣ���m1>m2��
H2S(g)+CO(g) ��H1=-17kJ/mol������ʼ�����n(H2)��n(COS)=m��mԽ����������Խ�࣬�����COS��ת���ʣ���m1>m2��
���¶ȸ���T0��COSת���ʼ�С�Ŀ���ԭ��Ϊ��i�и�Ӧ������ii.����������ͣ�iii.ƽ��������У�
(5)�ڳ��д����ĺ�ѹ�ܱ������н��з�Ӧ��.COS(g)+H2O(g)![]() H2S(g)+CO2(g) ��H2
H2S(g)+CO2(g) ��H2
������ӦΪ���ȷ�Ӧ������ƽ��������У���ѧƽ�ⳣ����С����M���Ӧ��ƽ�ⳣ��<Q���ƽ�ⳣ����
��N��COSת����Ϊ60%��COS(g)��H2O(g)Ͷ�ϱ�1��1��
COS(g)+H2O(g)![]() H2S(g)+CO2(g)
H2S(g)+CO2(g)
��ʼ��(mol) 1 1 0 0
�仯��(mol)0.6 0.6 0.6 0.6
ƽ����(mol) 0.4 0.4 0.6 0.6![]()
��Ӧ��ƽ��������COS(g)���ʵ�������![]() =20%��
=20%��
��M��COSת����Ϊ60%��COS(g)��H2O(g)Ͷ�ϱ�1��3��N��COSת����Ϊ60%��COS(g)��H2O(g)Ͷ�ϱ�1��1����Ӧ��������ʵ�����ͬʱ����Ӧǰ���������ʵ������䣬��M���Q���Ӧ��ƽ��������������ʵ���֮��Ϊ1��1��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£��������ݻ���Ϊ2.0 L�ĺ����ܱ������з�����Ӧ��2NO(g)��2CO(g)![]() N2(g)��2CO2(g)������������ʼ���ʵ����뷴Ӧ�¶����±���ʾ����Ӧ�����мס���������CO2�����ʵ�����ʱ��仯��ϵ��ͼ��ʾ��
N2(g)��2CO2(g)������������ʼ���ʵ����뷴Ӧ�¶����±���ʾ����Ӧ�����мס���������CO2�����ʵ�����ʱ��仯��ϵ��ͼ��ʾ��
���� | �¶�/�� | ��ʼ���ʵ���/mol | |
NO (g) | CO (g) | ||
�� | T1 | 0.20 | 0.20 |
�� | T1 | 0.30 | 0.30 |
�� | T2 | 0.20 | 0.20 |
����˵����ȷ����
A. �÷�Ӧ������ӦΪ���ȷ�Ӧ
B. �ﵽƽ��ʱ������CO2����������ȼ��е�С
C. T1��ʱ������ʼʱ����г���0.40 mol NO��0.40mol CO��0.40mol N2��0.40mol CO2����Ӧ�ﵽ��ƽ��ǰv(��)��v(��)
D. T2��ʱ������ʼʱ����г���0.06molN2��0.12 molCO2�����ƽ��ʱN2��ת���ʴ���40%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1mol N2O4��������ݻ�Ϊ10L���ܱ������У��ش��������⣺

��1��100 ��ʱ����ϵ�и�����Ũ����ʱ��仯����ͼ��ʾ����0~60 sʱ�Σ���Ӧ����v(N2O4)Ϊ________________����ӦN2O4(g)![]() 2NO2(g)��ƽ�ⳣ��KΪ___________��
2NO2(g)��ƽ�ⳣ��KΪ___________��
��2������Ӧ�ﵽƽ��ʱ�����ڷ�ӦN2O4(g)![]() 2NO2(g)���ı�ijһ����������˵���У�һ����˵����ѧƽ��������Ӧ�����ƶ�����____________��
2NO2(g)���ı�ijһ����������˵���У�һ����˵����ѧƽ��������Ӧ�����ƶ�����____________��
��������ɫ����
��N2O4�������������
�ۺ��º�ѹ����He
�ܵ�λʱ��������N2O4��NO2�����ʵ���֮�ȵ���1��2
��3������ʼʱ����1molNO2���壬��������ƽ��2NO2(g)![]() N2O4(g)�����NO2��ת����Ϊa%�����¶ȡ��������ʱ���ٳ���1molNO2���壬���´ﵽƽ��ʱ�����NO2��ת����Ϊb%����a______b������>������<������=�����������º�ѹʱ������1molNO2���壬��Ӧ2NO2(g)
N2O4(g)�����NO2��ת����Ϊa%�����¶ȡ��������ʱ���ٳ���1molNO2���壬���´ﵽƽ��ʱ�����NO2��ת����Ϊb%����a______b������>������<������=�����������º�ѹʱ������1molNO2���壬��Ӧ2NO2(g)![]() N2O4(g)�ﵽƽ�������������ͨ��һ������NO2���壬���´ﵽƽ��ʱ��NO2���������_________����������������������������С������
N2O4(g)�ﵽƽ�������������ͨ��һ������NO2���壬���´ﵽƽ��ʱ��NO2���������_________����������������������������С������
��4������ͼa��ʾ����ͨ�IJ���ƿ�г���NO2���壬��������ƽ��2NO2(g)![]() N2O4(g)����֪Fe3+��H2O2�ķֽ���д����ã�����ͼb��c�е���Ϣ���Ʋ�Aƿ��������ɫ��Bƿ�е�____________��������������dz������
N2O4(g)����֪Fe3+��H2O2�ķֽ���д����ã�����ͼb��c�е���Ϣ���Ʋ�Aƿ��������ɫ��Bƿ�е�____________��������������dz������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������20mL��0.10mol��L��1Cr2+��0.10mol��L��1Fe2+�Ļ����Һ�еμ�0.10 mol��L ��1NaOH��Һ������������Ũ�������NaOH��Һ����Ĺ�ϵ������ͼ��ʾ������Һ�н�������Ũ��С��10��5mol��L��1��Ϊ��ȫ������������˵���������
[Cr(OH)2��kspΪ2��10��16��Fe(OH)2��kspΪ8��10��16]

A. ����A��ʾc(Fe2+)
B. ��V(NaOH)=30mLʱ��Fe2+��ʼ����
C. ��pH=7ʱ��Һ��Fe2+��Cr2+����ȫ����
D. V(NaOH)>30mLʱ����Һ��c(Fe2+)�Uc(Cr2+) =4.0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ᾧ�����ɿ���H2C2O4��xH2O��ʾ��Ϊ�˲ⶨxֵ����������ʵ�飺
�ٳ�ȡw g���ᾧ�壬���100.00mLˮ��Һ��
����ȡ25.00mL�����ƵIJ�����Һ������ƿ�ڣ���������ϡ���ᡣ
����Ũ��Ϊa mol��L1��KMnO4��Һ�ζ����������һ��KMnO4����Ӻ�����ɫΪֹ��
��������Ӧ��KMnO4��H2C2O4��H2SO4����K2SO4��CO2����MnSO4��H2O��δ��ƽ����
�Իش�
��1��ʵ���в���Ҫ��������_____������ţ�����ȱ�ٵ�������_____��
a��������ƽ�������룬���ӣ���b���ζ��ܣ�c��100 mL��Ͳ��d���ζ��ܼУ�e���ձ���f��©����g����ƿ��h����������i��ҩ�ף�f����̨��
��2��ʵ���У���ҺKMnO4��ҺӦװ��_________ʽ�ζ����С�
��3��������ۣ�
�����ζ��յ�ʱ���ӵζ��̶ܿȣ����ɴ˲�õ�xֵ��_____(�ƫ��ƫС�����䡱����ͬ)��
�����ζ�ʱ���õ�����KMnO4��Һ����ö�����Ũ�ȱ�С�����ɴ˲�õ�xֵ��_____��
��4���ڵζ�����������a mol��L1��KMnO4��ҺV mL���������ƵIJ�����Һ�����ʵ���Ũ��Ϊ_________mol��L1���ɴ˿ɼ���xֵ��_________�����ô���ʽ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������ֺ��϶࣬���ȵ�������ڷ����µ���Ա����Ҫ�Ĵ�ʩ�Ǹ���Ա������������ͼ��ҽԺ����Ա��Һʱ�õ�һƿ��������Ϊ5%��������(C6H12O6)ע��Һ��ǩ��������۲��ǩ�����е����ݺ���д��

��1�������ǵ�Ħ������Ϊ________��
��2������Һ�к�ˮ________ g��
��3������Һ�����ʵ���Ũ��Ϊ________ mol/L(��ȷ��С���������λ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(G)������ֹѪ���ᣬ��һ���ѱ��㷺ʹ�ð�����͵�ֹѪҩ������һ�ֺϳ�·������(���ַ�Ӧ�������Լ���)��

�ش��������⣺
(1)A�Ľṹ��ʽ��___________��
(2)C�Ļ�ѧ������___________��
(3)�ڵķ�Ӧ������___________��
(4)E�в���Nԭ�ӵĹ���������Ϊ___________��
(5)������(G)�ķ���ʽΪ___________��
(6)д��������������������E��ͬ���칹��Ľṹ��ʽ��______________________��
a������ b.���������;�����ͬ������ c.�˴Ź��������������
(7)���![]() ��
�� Ϊԭ�ϣ��Ʊ�ҽҩ�м���
Ϊԭ�ϣ��Ʊ�ҽҩ�м��� �ĺϳ�·�ߣ�_______________________________________________________(���Լ���ѡ)��
�ĺϳ�·�ߣ�_______________________________________________________(���Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ������(��Ҫ����ͭ����������������)����ɫ����ұ����������Ҫ����������Դ�����������������ʵ����Դ���ۺ����þ�����Ҫ���塣һ�ִ�ͭ�������з�����ȡ���ֽ���Ԫ�صĹ����������£�

��֪���ֽ�Һ����Ҫ�ɷ�Ϊ[AuCl4]�����ֽ�������Ҫ�ɷ�ΪAgCl������Һ����Ҫ�ɷ�Ϊ[Ag(SO3)2]3�����Ҵ���[Ag(SO3)2]3��=Ag++2SO32��
(1)����ͭ��ʱ������ͭ������Ӧ�Ļ�ѧ����ʽΪ___________����֪����ͭ��ʱ��Ԫ�صĽ��������±���ʾ��

����ͭ��ʱ����������NaC1����Ҫ����Ϊ______________________��
(2)���ֽ���ʱ�����ʽ�����Ӧ�����ӷ���ʽΪ______________________��
(3)Na2SO3��Һ�к��������ʵ���������pH�Ĺ�ϵ��ͼ��ʾ��

��������ʱ����������������Һ��pH=4�������ܹ�����AgC��ԭ��Ϊ___________��������Һ��pH���ܹ��ͣ�����Ϊ___________��
(4)��֪����Ũ����10��5mol/Lʱ����Ϊ�����ӳ�����ȫ����֪�� Ksp[Pb(OH)2]=2.5��10��16��Ksp[Sb(OH)3]=10��41����ȡ�����������ɵõ���0.025 mol/L Pb2+����Һ(������Sb3+����)������ýϴ�����Pb2+��Һ������PH�ķ�ΧΪ___________��(������Һ����仯)
(5)��ҵ�ϣ�����Ϊ���������0.1 mol/L NiCl2��Һ��һ����NH4Cl��ɵĻ����Һ���ɵøߴ��ȵ����γ�ϸ���ۡ�����������һ��ʱ��NH4Cl��Ũ�ȶ���������Ч�ʼ����ijɷ��ʵ�Ӱ����ͼ��ʾ��

Ϊ����{���ȵ����γ�ϸ���ۣ�NH4Cl��Һ��Ũ����ÿ���Ϊ___________g/L����NH4Cl��Һ��Ũ�ȴ���15g/Lʱ����������ɫ��ζ�������ɣ�������������Ч�ʽ��ͣ�������Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���0.01molL-1��NaOH��Һ�ζ�20mLͬŨ�ȵ�HCN��Һ���ζ�������ͼ��ʾ������˵����ȷ���ǣ�������

A. Ka(HCN)��������Ϊ10-8
B. ���ʱ������Һ�е��뼸��1mol/L��HCN��Һ����Һ��c(H+)/ c(HCN)��ֵ����
C. ���ʱ����Һ����Ũ�ȴ�С��ϵ��c(HCN)>c(Na+)>c(CN-)>c(OH-)>c(H+)
D. �ڱ�ʾ����������У�ˮ�ĵ���̶������Ǣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com