
 NH2COONH4(s) ��H��0
NH2COONH4(s) ��H��0 

 2NH3 ��+ CaCl2 + 2H2O��
2NH3 ��+ CaCl2 + 2H2O��

 ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ�� ��� | ��Ӧ�¶� (����ˮԡ����) | ϡ���� ��Һ | MnSO4���� | 0.10 mol/L KMnO4��Һ | ��ɫ ʱ�� |
| 1 | 750C | һ | һ | 1mL | 100s |
| 2 | 750C | 10�� | һ | 1mL | 40s |
| 3 | 750C | 10�� | �������� | 1mL | 3s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ��� | ʵ����� | ʵ����������� | �� �� |
| �� | �� ��a g M�м���һ����ϡ���ᣬ��ֽ��裻 �� �����μ�ϡ����������, ��ַ�Ӧ. | �ٹ������Լ��٣� ����Ȼ��һ�������壬��Һ����ɫ | ��M��һ����Cu2O; ��M��һ����Cu. |
| �� | ����ʵ���������Һ���� ������ϴ�ӡ�������� | ��������Ϊ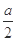 g g | MΪCu��Cu2O�Ļ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
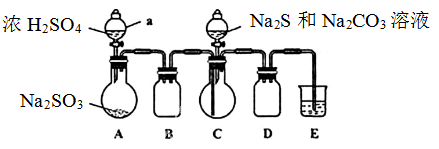
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
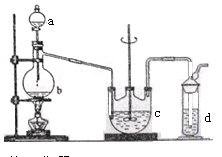
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ζ���� | ����Һ�����(mL) | ���������Һ�����(mL) | |
| ��ʼ���� �ζ�ǰ | �յ���� | ||
| 1 | 20.00 | 0.50 | 20.40 |
| 2 | 20.00 | 6��00 | 26��10 |
| 3 | 20.00 | 4��00 | 24��00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
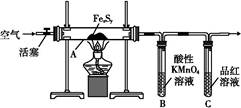
| �ζ����� | ������Һ ���/mL | ������Һ���/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 1.50 | 23.70 |
| 2 | 25.00 | 1.02 | 26.03 |
| 3 | 25.00 | 0.00 | 24.99 |
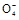 +2H2O+5SO2
+2H2O+5SO2 2Mn2++5S
2Mn2++5S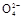 +4H+
+4H+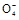 +6H++5H2C2O4
+6H++5H2C2O4 2Mn2++10CO2��+8H2O
2Mn2++10CO2��+8H2O| ��� | �¶�/�� | �ữ��H2C2O4 ��Һ/mL | KMnO4 ��Һ/mL | ��Һ�� ɫʱ��/s |
| 1 | 25 | 5.0 | 2.0 | 40 |
| 2 | 25 | 5.0(������������ ��ˮ��MnSO4��ĩ) | 2.0 | 4 |
| 3 | 60 | 5.0 | 2.0 | 25 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com