
| ʵ���� | ��Ӧ�� | ���� |
| �� | 10mL2% H2O2��Һ | �� |
| �� | 10mL5% H2O2��Һ | �� |
| �� | 10mL5% H2O2��Һ | 1mL0.1mol•L-1FeCl3��Һ |
| �� | 10mL5% H2O2��Һ+����HCl��Һ | 1mL0.1mol•L-1FeCl3��Һ |
| �� | 10mL5% H2O2��Һ+����NaOH��Һ | 1mL0.1mol•L-1FeCl3��Һ |
���� ��1�������ı䷴Ӧ��;�������ͷ�Ӧ����Ļ�ܣ�
��2��pHԼΪ6����������������ӣ���Һ�����ԣ�
��3���Ƚ϶��գ�����ͬ��������ʵ��ٺ͢�H2O2��Һ��Ũ�Ȳ�ͬ��ʵ��Ŀ����̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죻
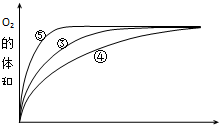
��4����ͼ��֪���ݵķ�Ӧ������ܵķ�Ӧ������С������ͼ����жϣ����Ի���������H2O2�ֽ�����ʣ����Ի����ܼ�СH2O2�ֽ�����ʣ�
��� �⣺��1�����ڴ����ı��˷�Ӧ��;�������ͷ�Ӧ����Ļ�ܣ��Ӷ��ӿ췴Ӧ���ʣ�
�ʴ�Ϊ�������˻�ܣ�
��2��������5%H2O2��Һ��pHԼΪ6����Һ�����ԣ�˵��˫��ˮ���Կ��������ᣬ�������뷽��ʽΪH2O2?H++HO2-��
�ʴ�Ϊ��H2O2?H++HO2-��
��3��ʵ���10mL2% H2O2��Һ��ʵ���10mL5% H2O2��Һ��������˫��ˮ��Ũ�Ȳ�ͬ������ʵ���Ŀ�������ʵ���Ŀ��Ϊ̽��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬
�ʴ�Ϊ��̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죻
��4��ʵ��ۡ��ܡ����в�ͬ������Һ������ԣ���ͼ��֪���ݵķ�Ӧ������ܵķ�Ӧ������С�����ʵ�鷽����֪�����Ի���������H2O2�ֽ�����ʣ����Ի����ܼ�СH2O2�ֽ�����ʣ�
�ʴ�Ϊ�����Ի���������H2O2�ֽ�����ʣ����Ի����ܼ�СH2O2�ֽ�����ʣ�
���� ���⿼��Ӱ�컯ѧ��Ӧ���ʵ����أ��ϺõĿ���ѧ��ʵ����ơ����ݴ�����ͼ��������ۺ��������Ѷ��еȣ�ע����Ϣ�����ü��ɽ��


 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2O2��CO2 | B�� | Na��O2 | C�� | NaOH��CO2 | D�� | NaAlO2��HNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | NH4Al��SO4��2��Һ�����NaOH��Һ��Ӧ��Al3++4OH-�TAlO2-+2H2O | |
| B�� | ICl�������ϡKOH��Һ�У�ICl+2OH-�TCl-+IO-+H2O | |
| C�� | �ö��Ե缫���CuSO4��Һ��2Cu2++4OH-$\frac{\underline{\;���\;}}{\;}$2Cu��+O2��+2H2O | |
| D�� | NaAlO2��Һ��AlO2-��ˮ�⣺AlO2-+2H2O�TAl��OH��3+OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���ᣨH2C2O4����������Ȼ���ֲ���У���K1=5.4��10-2��K2=5.4��10-5�����л�ԭ�ԣ�����ˮ����Һ�����ԣ�Ϊ�ⶨijH2C2O4��Һ��Ũ�ȣ�ȡ����Һ����ƿ�У���������ϡH2SO4����Ũ��Ϊc mol/L KMnO4����Һ�ζ����ζ�ԭ��Ϊ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O
���ᣨH2C2O4����������Ȼ���ֲ���У���K1=5.4��10-2��K2=5.4��10-5�����л�ԭ�ԣ�����ˮ����Һ�����ԣ�Ϊ�ⶨijH2C2O4��Һ��Ũ�ȣ�ȡ����Һ����ƿ�У���������ϡH2SO4����Ũ��Ϊc mol/L KMnO4����Һ�ζ����ζ�ԭ��Ϊ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����KMnO4��Һ���/mL | 22.32 | 24.39 | 24.41 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
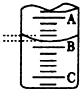
| ��ƿ����Һ | �ζ�������Һ | ѡ��ָʾ�� | ѡ�õζ��� | |
| A | �� | �� | ʯ�� | ���ң� |
| B | �� | �� | ���� | ���ף� |
| C | �� | �� | ��̪ | ���ף� |
| D | �� | �� | ʯ�� | ���ң� |
| ������������ | 0.100mol/L�������� | ||
| �ζ����� | ��Һ�������mL�� | �ζ�ǰ�Ŀ̶ȣ�mL�� | �ζ���Ŀ̶ȣ�mL�� |
| ��һ�� | 25.00 | 1.68 | 26.89 |
| �ڶ��� | 25.00 | 0.00 | 27.91 |
| ������ | 25.00 | 0.12 | 25.01 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������̼ͨ�롰ˮ�������У�C02+SiO32-+H20�TC032-+H2SiO3�� | |
| B�� | ��ˮ���� AlCl3 ��Һ�У�Al3++30H-�TAl��0H��3�� | |
| C�� | ���ܽ���NaOH��Һ�У�2Al+2OH-+6H2O�T2[Al��OH��4]-+3H2�� | |
| D�� | Al2O3���� NaOH ��Һ�У�Al2O3+2OH-+3H2O=2[Al��0H��4]- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| A | C | |
| B |
 ��C�ĵ�����H2��Ӧ�Ļ�ѧ����ʽΪ��H2+F2=2HF
��C�ĵ�����H2��Ӧ�Ļ�ѧ����ʽΪ��H2+F2=2HF�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
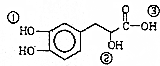
| A�� | ���л���ķ���ʽΪC9H10O5 | |
| B�� | ���л����ܷ������ۡ��ӳɡ���ȥ��������Ӧ | |
| C�� | 1mol���л��������Ժ�4molNaOH������Ӧ | |
| D�� | ���л�������Т١��ڡ���3��-OH��������ǿ������˳���Ǣۣ��٣��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
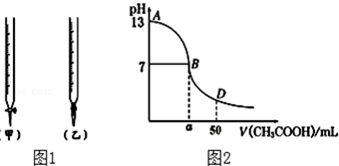
| A�� | c��H+�� | B�� | c��H+��•c��OH-�� | C�� | $\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$ | D�� | $\frac{c��O{H}^{-}��}{c��{H}^{+}��}$ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com