
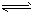 H++A-�������ƽ�ⳣ��
H++A-�������ƽ�ⳣ��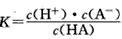 ��Kֻ���¶��йأ�cΪ������ƽ��Ũ�ȡ��±���25��ʱ���ֳ�������ĵ���ƽ�ⳣ����
��Kֻ���¶��йأ�cΪ������ƽ��Ũ�ȡ��±���25��ʱ���ֳ�������ĵ���ƽ�ⳣ���� 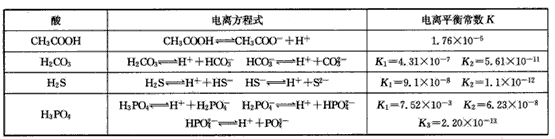
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| C(A-)��C(H+) |
| C(HA) |
| C(A-)��C(H+) |
| C(HA) |
| �� | ���뷽��ʽ | ����ƽ�ⳣ��K |
| CH3COOH | CH3COOH?CH3COOH-+H+ | 1.76��10-5 |
| H2CO3 | H2CO3?H++HCO3- HCO3-?H++HCO32- |
K1=4.31��10-7 K2=5.61��10-11 |
| H2S | H2S?H++HS- HS-?H++S2- |
K1=9.1��10-8 K2=1.1��10-12 |
| H3PO4 | H3PO4?H++H2PO4- H2PO4-H++HPO42- HPO42-?H++PO43- |
K1=7.52��10-3 K2=6.23��10-8 K3=2.20��10-13 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �� | ���뷽��ʽ | ����ƽ�ⳣ��K |
| CH3COOH | CH3COOH?CH3COO-+H+ | 1.76��10-5 |
| HClO | HClO?ClO-+H+ | 2.95��10-8 |
| H2S | H2S?H++HS- HS-?H++S2- |
K1=9.1��10-8 K2=1.1��10-12 |
| H2CO3 | H2CO3?H++HCO3- HCO3-?H++CO32- |
K1=4.31��10-7 K2=5.61��10-11 |
| H3PO4 | H3PO4?H++H2PO4- H2PO4-?H++HPO42- HPO42-?H++PO43- |
K1=7.1��10-3 K2=6.3��10-8 K3=4.2��10-13 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ᣬ��һ���¶��´ﵽ����ƽ��ʱ��������Ũ�ȴ���һ�ֶ����Ĺ�ϵ���±���25��ʱ���ֳ�������ĵ���ƽ�ⳣ��
| �� | ���뷽��ʽ | ����ƽ�ⳣ��K |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ᣬ��һ���¶��´ﵽ����ƽ��ʱ��������Ũ�ȴ���һ�ֶ����Ĺ�ϵ���±���25��ʱ���ֳ�������ĵ���ƽ�ⳣ��
| �� | ���뷽��ʽ | ����ƽ�ⳣ��K |
| | | |
| | | |
| | | |
| | | |
�ش����и��ʣ�
��1��Kֻ���¶��йأ����¶�����ʱ��Kֵ________�����������С���������䡱����
��2�����¶���ͬʱ���������Kֵ��ͬ����ôKֵ�Ĵ�С�����Ե����ǿ���кι�ϵ?__________________��
��3������CH3COOH��H2CO3��HCO3-��H2S��HS-��H3PO4��H2PO4-��HPO42-���������ᣬ����������ǿ����_________����������________��
��4����Ԫ�����Ƿֲ�����ģ�ÿһ��������Ӧ�ĵ���ƽ�ⳣ��������ͬһ�ֶ�Ԫ�����K1��K2��K3֮������������ϵĹ��ɣ�����H3PO4�˹�����________________�������˹��ɵ�ԭ����_________________________��
![]() ��5������ƽ�ⳣ������ʵ��ķ����ⶨ�����ģ����Ѿ����ij�¶��� NH3∙H2O��Һ�д������·�Ӧ��NH3∙H2O NH4++OH- ��֪0.10 mol��L-1NH3∙H2O��Һ�У��ﵽƽ��ʱ��Cƽ����OH-��=4.2 �� 10-3mol��L-1��Cƽ����NH3∙H2O����C��ʼ��NH3∙H2O����ˮ�ĵ���ɺ��Բ���;
��5������ƽ�ⳣ������ʵ��ķ����ⶨ�����ģ����Ѿ����ij�¶��� NH3∙H2O��Һ�д������·�Ӧ��NH3∙H2O NH4++OH- ��֪0.10 mol��L-1NH3∙H2O��Һ�У��ﵽƽ��ʱ��Cƽ����OH-��=4.2 �� 10-3mol��L-1��Cƽ����NH3∙H2O����C��ʼ��NH3∙H2O����ˮ�ĵ���ɺ��Բ���;
����pH��ֽ������Һ��pHֵ���������Cƽ����OH-�����ⶨ��ҺpHֵ�IJ�����______________��
�ڲ���Cƽ����NH3∙H2O���ķ��������_____________����������ƣ�
������¶��¸÷�Ӧ��ƽ�ⳣ��K.(д��������̣�����������2λ��Ч����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ�߶���ѧ�����п��Ի�ѧ�� ���ͣ������
�������ᣬ��һ���¶��´ﵽ����ƽ��ʱ��������Ũ�ȴ���һ�ֶ����Ĺ�ϵ���±���25��ʱ���ֳ�������ĵ���ƽ�ⳣ��
|
�� |
���뷽��ʽ |
����ƽ�ⳣ��K |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
�ش����и��ʣ�
��1��Kֻ���¶��йأ����¶�����ʱ��Kֵ________�����������С���������䡱����
��2�����¶���ͬʱ���������Kֵ��ͬ����ôKֵ�Ĵ�С�����Ե����ǿ���кι�ϵ?__________________��
��3������CH3COOH��H2CO3��HCO3-��H2S��HS-��H3PO4��H2PO4-��HPO42-���������ᣬ����������ǿ����_________����������________��
��4����Ԫ�����Ƿֲ�����ģ�ÿһ��������Ӧ�ĵ���ƽ�ⳣ��������ͬһ�ֶ�Ԫ�����K1��K2��K3֮������������ϵĹ��ɣ�����H3PO4�˹�����________________�������˹��ɵ�ԭ����_________________________��
 ��5������ƽ�ⳣ������ʵ��ķ����ⶨ�����ģ����Ѿ����ij�¶��� NH3∙H2O��Һ�д������·�Ӧ��NH3∙H2O NH4++OH-
��֪0.10 mol��L-1
NH3∙H2O��Һ�У��ﵽƽ��ʱ��Cƽ����OH-��=4.2 �� 10-3mol��L-1��Cƽ����NH3∙H2O����C��ʼ��NH3∙H2O����ˮ�ĵ���ɺ��Բ���;
��5������ƽ�ⳣ������ʵ��ķ����ⶨ�����ģ����Ѿ����ij�¶��� NH3∙H2O��Һ�д������·�Ӧ��NH3∙H2O NH4++OH-
��֪0.10 mol��L-1
NH3∙H2O��Һ�У��ﵽƽ��ʱ��Cƽ����OH-��=4.2 �� 10-3mol��L-1��Cƽ����NH3∙H2O����C��ʼ��NH3∙H2O����ˮ�ĵ���ɺ��Բ���;
����pH��ֽ������Һ��pHֵ���������Cƽ����OH-�����ⶨ��ҺpHֵ�IJ�����______________��
�ڲ���Cƽ����NH3∙H2O���ķ��������_____________����������ƣ�
������¶��¸÷�Ӧ��ƽ�ⳣ��K.(д��������̣�����������2λ��Ч����)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com