
| A�� | ����$\left\{\begin{array}{l}{�����}\\{������\left\{\begin{array}{l}{��Һ}\\{������}\end{array}\right.}\end{array}\right.$ | |
| B�� | ������$\left\{\begin{array}{l}{����\left\{\begin{array}{l}{��������}\\{�ǽ�������}\end{array}\right.}\\{������}\end{array}\right.$ | |
| C�� | ������$\left\{\begin{array}{l}{���}\\{����\left\{\begin{array}{l}{��\left\{\begin{array}{l}{������}\\{��������}\end{array}\right.}\\{��}\\{��}\end{array}\right.}\end{array}\right.$ | |
| D�� | ������$\left\{\begin{array}{l}{����\left\{\begin{array}{l}{����}\\{������}\\{������}\end{array}\right.}\\{���}\end{array}\right.$ |
���� ��״���෨��һ�ֺ�����ķ��෨�����ղ�Σ�һ��һ�����֣�����һ�ô�������Ҷ��֦���ˡ���������֮���ǰ����ͱ������Ĺ�ϵ��
��� �⣺A��������������ʺͻ����������Һ�ͻ������Һ���ڻ�����A����
B��������������ʺͻ���������ְ����������ʺͷǽ����������࣬��B��ȷ��
C��������������ᡢ��Ρ�������������������������ͷǽ������������֣���C����
D���л���������Ǵ������л�����ķ��룬��D����
��ѡB��
���� ���⿼�����ʵķ����Լ���������֮��Ĺ�ϵ���ѶȲ���ƽʱע��֪ʶ�Ļ��ۣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1 mol•L-1FeCl3��Һ�У�K+��Na+��AlO2-��SCN- | |
| B�� | ���ܽ�CaCO3����Һ�У�Fe2+��Ca2+��Cl-��NO${\;}_{{3}_{\;}^{\;}}^{-}$ | |
| C�� | ����������Һ�У�Cu2+��Mg2+��SO${\;}_{4}^{2-}$��Cl- | |
| D�� | ��ˮ����õ���c��H+��=1��10-13mol•L-1����Һ�У�Mg2+��Cu2+��SO42-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1��9 | B�� | 1��1 | C�� | 1��2 | D�� | 1��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
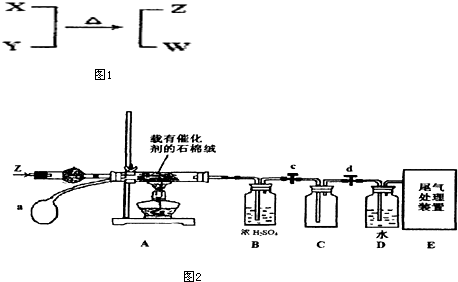
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȡ1.8g�������ƹ��壬����450mLˮ | |
| B�� | ��ȡ1.8g�������ƹ������450mL��Һ | |
| C�� | ��ȡ2.0g�������ƹ��壬����500mLˮ | |
| D�� | ��ȡ2.0g�������ƹ������500mL��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaAlO2��Һ��ͨ������������̼��2AlO2-+CO2+3H2O�T2Al��OH��3��+CO32- | |
| B�� | NaOH��Һ��ͨ������������̼��2OH-+CO2�TCO32-+H2O | |
| C�� | AlCl3��Һ�м��������ˮ��Al3++3NH3•H2O�TAl��OH��3��+3NH4+ | |
| D�� | AlCl3��Һ�м������NaOH��Һ��Al3++3OH-�TAl��OH��3�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com