
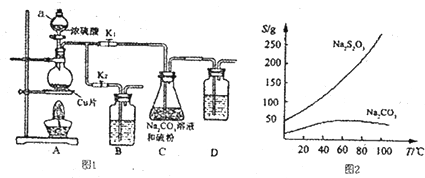
���� ��1������1���������������������ʣ�����װ�������ԣ�
����2��װ��B��D�������ǽ���β����������ֹβ���ж���������Ⱦ������
����3�������������������Һ�в��ȶ���Ӧ������ҺΪ�����ԣ���������ҺpH�ӽ���С��7��
����4������Һ�л�þ��壬��Ҫ����Ũ�������ȹ��ˣ��ٽ���Һ��ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��
��2������Ŀ��Ϣ��֪��Na2S2O3����ˮ������Ӧ����Na2SO4��H2SO4����������ԭΪHCl��
��3���������֪��BaCrO4�������ܽ�ת��ΪCr2O2-7����Ԫ���غ㼰��֪����ʽ�ɵù�ϵʽ��2Ba2+��2BaCrO4��Cr2O2-7��3I2��6Na2S2O3��������ĵ�Na2S2O3���ù�ϵʽ������Һ��n��Ba2+������������c��Ba2+����
��� �⣺��1������1���������������������ʣ�����װ�������ԣ��������Ϊ���ر�K2��K1����D�м�ˮ��û����ĩ�ˣ�����ë����˫����ס��ƿ��D�е���������ð������ȴ���γ�1��ˮ����˵�����������ã�
�ʴ�Ϊ���ر�K2��K1����D�м�ˮ��û����ĩ�ˣ�����ë����˫����ס��ƿ��D�е���������ð������ȴ���γ�1��ˮ����˵�����������ã�
����2��װ��B��D�������ǽ���β����������ֹβ���ж���������Ⱦ����������������л�ԭ�ԣ����������Ը��������Һ�������գ�����������������������Һ��̼��������Һ��Ӧ�����գ���ѡ��ACD��
����3�������������������Һ�в��ȶ���Ӧ������ҺΪ�����ԣ����Կ�����ҺpH�ӽ���С��7��
����4������Һ�л�þ��壬��Ҫ����Ũ�������ȹ��ˣ��ٽ���Һ��ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��
�ʴ�Ϊ����ȴ�ᾧ��
��2������Ŀ��Ϣ��֪��Na2S2O3����ˮ������Ӧ����Na2SO4��H2SO4����������ԭΪHCl����Ӧ����ʽΪ��Na2 S2O3+4Cl2+5H2O=Na2SO4+H2SO4+8HCl��
�ʴ�Ϊ��Na2S2O3+4Cl2+5H2O=Na2SO4+H2SO4+8HCl��
��3���������֪��BaCrO4�������ܽ�ת��ΪCr2O2-7����Ԫ���غ㼰��֪����ʽ�ɵù�ϵʽ��2Ba2+��2BaCrO4��Cr2O2-7��3I2��6Na2S2O3�����ĵ�Na2S2O3Ϊ0.018L��0.01mol/L����n��Ba2+��=0.018L��0.01mol/L��$\frac{1}{3}$=0.00006mol������Һ��c��Ba2+��=$\frac{0.00006mol}{0.025L}$=0.0024mol/L��
�ʴ�Ϊ��0.0024mol/L��
���� ���⿼��ʵ���Ʊ�������ƣ��漰�����Լ��顢��ʵ��װ�ü�����ķ������ۡ����ʵķ����ᴿ��������ԭ��Ӧ�ζ�����3����ע�����ù�ϵʽ���м��㣬�Ѷ��еȣ�


 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
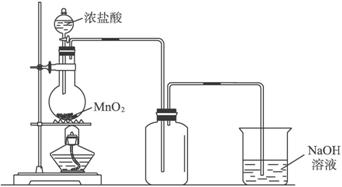

| ��ҺA | ����B | ����C | |
| a | ϡ���� | Zn | Cl2 |
| b | Ũ���� | MnO2 | H2 |
| c | ϡ���� | Fe | Cl2 |
| d | Ũ���� | KMnO4 | H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
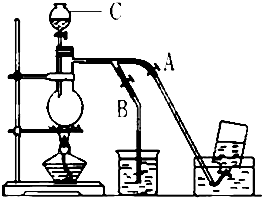 ��ͼ��ʾ��û��ͨ������������Ʊ�����ʱ��Ƶ�װ�ã�ͼ��a��b�ǿɿ��Ƶĵ������У��������ڱ����Ȼ�����Һ�е��ܽ�Ƚ�С����
��ͼ��ʾ��û��ͨ������������Ʊ�����ʱ��Ƶ�װ�ã�ͼ��a��b�ǿɿ��Ƶĵ������У��������ڱ����Ȼ�����Һ�е��ܽ�Ƚ�С�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ѡ�� | �� | �� | �� |
| A | �������ɫ���� | ��ʯ�� | ʪ�����ɫ���� |
| B | �������ɫ���� | �轺 | ʪ�����ɫ���� |
| C | ʪ�����ɫ���� | Ũ���� | �������ɫ���� |
| D | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Q1=Q2=Q3 | B�� | Q2��Q1��Q3 | C�� | Q2��Q3��Q1 | D�� | Q2=Q3��Q1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��ʼ������pH | ��ȫ������pH | |
| Fe3+ | 1.1 | 3.2 |
| Fe2+ | 5.8 | 8.8 |
| Zn2+ | 5.9 | 8.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ͻ������ٺ������ֽ��� | |
| B�� | Ư�����ڿ����г��ڴ�� | |
| C�� | ����ʦ����������ʴʯӢ��������Ʒ | |
| D�� | NaOH��Һ����������ĥ�ڲ�������ϸ���Լ�ƿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | p��Ne����p��N2����p��O2�� | B�� | p��O2����p��Ne����p��N2�� | C�� | p��N2����p��O2����p��Ne�� | D�� | p��N2����p��Ne����p��O2�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com