
ʳ���к���һ������þ���������ʣ��ӵ����е����ʧ��Ҫ���������ʡ�ˮ�֡������е������Լ����ա����ȶ�����ġ�
��֪���������ԣ�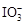 ��Fe3����I2����ԭ�ԣ�
��Fe3����I2����ԭ�ԣ�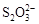 ��I������KI��I2
��I������KI��I2 KI3
KI3
��1��ijѧϰС��Լӵ��ν�������ʵ�飺ȡһ����ij�ӵ���(���ܺ���KIO3��KI��Mg2����Fe2����Fe3��)������������ˮ�ܽ⣬����ϡ�����ữ����������Һ��Ϊ4�ݡ���һ����Һ�еμ�KSCN��Һ���Ժ�ɫ���ڶ�����Һ�м�����KI���壬��Һ�Ե���ɫ����CCl4��ȡ���²���Һ���Ϻ�ɫ����������Һ�м�������KIO3����μӵ����Լ�����Һ����ɫ��
������ķ���Һ�м�K3Fe(CN)6��Һ�������Ƿ�õ�����������ɫ�ij������ɼ����Ƿ��� �������ӷ��ű�ʾ������ɫ�ij�����_______���û�ѧʽ��ʾ����
�ڵڶ�����Һ�м�������KI�����Ӧ�����ӷ���ʽΪ��______��______
��2��KI��Ϊ�ӵ����ʳ���ڱ�������У����ڿ��������������ã�������������ʧд����ʪ������KI��������Ӧ�Ļ�ѧ����ʽ��_________________��
��I2����KI��Һ���ڵ��������£����Ƶ�KI3��H2O�������ʲ��ʺ���Ϊʳ�μӵ������������_________________________________��
��3��ijͬѧΪ̽���¶Ⱥͷ�Ӧ��Ũ�ȶԷ�Ӧ2IO3����5SO32����2H��=I2��5SO42����H2O�����ʵ�Ӱ�죬���ʵ�����±���ʾ��
| | 0.01mol��L��1KIO3 ������Һ(������) �����/mL | 0.01mol��L��Na2SO3 ��Һ�����/mL | H2O�� ���/mL | ʵ�� �¶� /�� | ��Һ���� ��ɫʱ�� ��ʱ��/s |
| ʵ��1 | 5 | V1 | 35 | 25 | t1 |
| ʵ��2 | 5 | 5 | 40 | 25 | t2 |
| ʵ��3 | 5 | 5 | V2 | 0 | t3 |
����14�֣�
��1���� Fe2�� �� Fe 3[Fe(CN)6]2 ����1�֣�
��IO3����5I����6H��=3I2��3H2O 2Fe3����2I��=2Fe2����I2 ����2�֣�
��2��O2��4KI��2H2O=2I2��4KOH ��2�֣�
KI3�����ȣ���ʪ�������²���I2��KI��KI������������I2����������2�֣�
��3��< �� 40 ����2�֣�
�������������
��1����Fe2�������軯����Һ��������Ӧ����
��Ӧ����Ϣ���������ԣ�IO3����Fe3����I2����˵��IO3����Fe3����������I������I2��
��2��KI����ʪ����������������ʧ�����Կ���I�������������γ�I2�����Ӷ������ʧ��
������֪��Ϣ����KI��I2 KI3�����������ʲ��ȶ��ԣ������ֽ���̡�
KI3�����������ʲ��ȶ��ԣ������ֽ���̡�
��3���ɱ�����Ϣ��֪������ʵ��Ϊƽ�ж���ʵ�飬Ҫ��ѭ����һ�������Ĺ������������ʵ����Һ�������������50 ml�����V2="40" ml��ʵ��1��ʵ��2�Ƚϣ�ʵ��2��ˮ������࣬˵������Ũ�ȱ�ʵ��1�е�ҪС����Ӧ������������ʱ�䳤������t1��t2
���㶨λ�����������ӵļ��飬������ԭ��Ӧ����д��ʵ��������ݷ��������֪ʶ������ѧ�������Ϣ�Ķ�ȡ�ͷ�����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
д�����з�Ӧ�Ļ�ѧ����ʽ����ָ���������ͻ�ԭ����
(1)��ڿ�����ȼ��
(2)����ˮ��Ӧ
(3)þ���ڶ�����̼������ȼ��
(4)���������Ϊͭ����ʪ����ͭ
(5)�ӳ�������������������ҵұ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij����С��ѧ����Cl2��FeBr2��Һ��Ӧ����ʵ��̽����
| �� �� | �� �� |
| ȡ0��1 mol/L��FeBr2��Һ10 mL������Һ��pH | pH��ֽ��죨ԼΪ4�� |
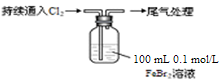 | ������ʼͨ��100 mL���ۺϱ�״��������Һ��dz��ɫ��ƣ� �����Ժ���ͨ��Cl2����Һ��ɫ�����Ϊ�ػ�ɫ�� iii���Ժ���Һ���ػ�ɫ��dz�����ձ�Ϊ��ɫ |
| �Թ� | ���� | ���� | ���� |
| a | ȡ2 mL FeCl2��Һ���μ�������ˮ��2��KSCN��Һ���� | | Cl2��������ǿ��Fe3+ |
| b | ȡ2 mL FeCl3��Һ���μ������廯����Һ������CCl4���� | CCl4��û�����Ա仯 | |
| c | | CCl4������ɫ��Ϊ�Ȼ�ɫ | Cl2��������ǿ��Br2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
KMnO4��Һ������������ԭ��Ӧ�ζ��ı�Һ������KMnO4��ǿ�����ԣ�������Һ�����ױ������л�ˮ�е�ijЩ������ԭ�����ʻ�ԭ����������������MnO(OH)2���������KMnO4����Һ�IJ���������ʾ���ٳ�ȡ�Զ�����������KMnO4��������ˮ�У�����Һ���Ȳ�������1 h�������ײ���©�����˳�ȥ���ܵ�MnO(OH)2���۹��˵õ���KMnO4��Һ��������ɫ�Լ�ƿ�����ڰ�����������������ԭ�ζ��������û��Լ�(���ȸߡ���Է��������ϴ��ȶ��ԽϺõ�����)��Һ�궨��Ũ�ȣ�KMnO4�ڵζ��б���ԭ��Mn2+����ش��������⣺
��1�� ȷ��ȡһ�������KMnO4��Һ��Ҫʹ�õ�������____________��
��2�� �����������У����ڱ궨KMnO4��Һ�Ļ��Լ����ѡ��________(�����)��
| A����������Ϊ30%��˫��ˮ | B��FeSO4 | C��Ħ���� | D��Na2SO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ��֤�����ԣ�Cl2��Fe3+��SO2��ijС������ͼ��ʾװ�ý���ʵ�飨�г�������A�еļ���װ�����ԣ��������Ѿ�������ϣ�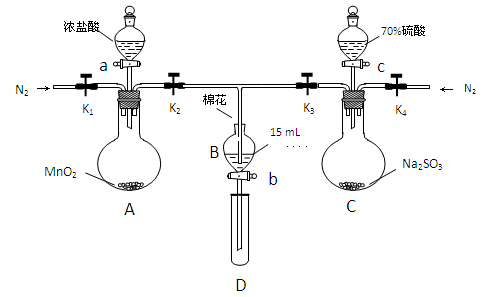
ʵ����̣�
���ɼ�K1-K4��ͨ��һ��ʱ��N2���ٽ�T�͵��ܲ���B�У�����ͨ��N2������ر�K1��K2��K3 .
����a���μ�һ������Ũ���ᣬ��A����.
��B�е���Һ���ʱ��ֹͣ���ȣ��н����ɼ�K2.
��������b��ʹԼ2ml����Һ����D�Թ��У��������е�����.
�������ɼ�K3������c������70%�����ᣬһ��ʱ���н����ɼ�K3.
��.�����Թ�D���ظ����̢�������B��Һ�е�����.
��1�����̢��Ŀ����___________________________
��2�����н������ҺΪ_______________________
��3��A�з�����Ӧ�Ļ�ѧ����ʽΪ_____________________________________________
��4����70%������֧ȡSO2����Ӧ���ʱ���98%������飬ԭ����___________________
��5�����̢��м���B��Һ���Ƿ���������IJ�����______________________________
��6���ס��ҡ�����λͬѧ�ֱ����������ʵ�飬���ǵļ����һ���ܹ�֤������Cl2��Fe3+��SO2����
| | ���̢� B��Һ�к��е����� | ���̢� B��Һ�к��е����� |
| �� | ��Fe3+��Fe2+ | ��SO42- |
| �� | ����Fe3+����Fe2+ | ��SO42- |
| �� | ��Fe3+��Fe2+ | ��Fe2+ |
 FeSO3��s��
FeSO3��s��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��14�֣���ijͬѧ�������ȷ�Ӧ����ʵ�顣���ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��Һ�п��ܺ���H+��NH4+��Mg2+��Al3+��Fe3+��CO32?��SO42?��NO3?�еļ��֡��ټ�����Ƭ��������ɫ��ζ�����壻�ڼ���NaOH��Һ��������ɫ�������Ҳ����ij����������NaOH�����ʵ���֮��Ĺ�ϵ����ͼ��ʾ��������˵����ȷ���ǣ�������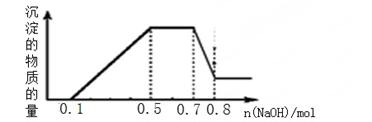
| A����Һ��һ������CO32?�����ܺ���SO42?��NO3? |
| B���ڵμ�NaOH��Һ���ʵ���Ϊ0��5��0��7molʱ�����������ӷ�ӦΪ��Al3+��4OH-��[Al(OH)4]- |
| C����Һ�е�������ֻ��H+��Mg2+��Al3+ |
| D��n(H+)��n(NH4+)��n(Mg2+) =2��4��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ͼ��ʾװ�������ﵽ�й�ʵ��Ŀ����
| A���ü�ͼװ��֤���ܶȦ�(ú��)����(��)����(ˮ)������ �� |
| B������ͼװ���Ʊ�Fe(OH)2 |
| C���ñ�ͼװ����ȡ������ |
| D���ö�ͼװ�ñȽ�NaHCO3��Na2CO3�����ȶ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪�������������ܷ������з�Ӧ��Cu���� Cu��Cu2��(δ��ƽ)��NH4CuSO3��������2mol��L-1������Һ����ȣ����������������Ϻ�ɫ�������ɣ����д̼�����ζ�������������Һ����ɫ���ݴ��ж�����˵��һ����������
| A���÷�Ӧ��ʾ����������� | B��NH4CuSO3����Ԫ�ر����� |
| C���̼�����ζ�������Ƕ������� | D����Ӧ�������������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com