
ĻĀĶ¼ŹĒ֊ѧ»Æѧ֊³£¼ūµÄŅ»Š©µ„ÖŹ»ņ»ÆŗĻĪļÖ®¼äµÄ×Ŗ»Æ¹ŲĻµĶ¼£¬ĘäÖŠ²æ·Ö·“Ó¦ÖŠÉę¼°µ½µÄĖ®Ī“ĮŠ³ö”£ŅŃÖŖA”¢B”¢FŹĒČĖĄąÉś“ę»ņČÕ³£Éś»īÖŠ±Ų²»æÉÉŁµÄĪļÖŹ£¬I”¢J¾ł¾ßÓŠĘư׊Ō£¬µ«Į½Õß»ģŗĻŗóµÄ²śĪļ¾ł²»¾ßÓŠĘư׊Ō”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Å AµÄ¾§ĢåĄąŠĶĪŖ £¬EµÄµē×ÓŹ½ĪŖ £»
¢ĘŠ“³öĻĀĮŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ·“Ó¦¢Ł £¬
·“Ó¦¢Ū ”£
¢ĒČō·“Ó¦¢ÜÖŠ1molĪļÖŹCĶźČ«·“Ó¦·Å³öa kJµÄČČĮ棬Š“³ö·“Ó¦¢ÜµÄČČ»Æѧ·½³ĢŹ½ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ



| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 »ÆѧŹµŃé×°ÖƵÄÕżČ·Į¬½ÓŹĒŹµŃé³É°ÜµÄ¹Ų¼ü£¬ĻĀĶ¼ŹĒ֊ѧ»Æѧ֊³£¼ūµÄŹµŃé×°ÖĆ£®
»ÆѧŹµŃé×°ÖƵÄÕżČ·Į¬½ÓŹĒŹµŃé³É°ÜµÄ¹Ų¼ü£¬ĻĀĶ¼ŹĒ֊ѧ»Æѧ֊³£¼ūµÄŹµŃé×°ÖĆ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
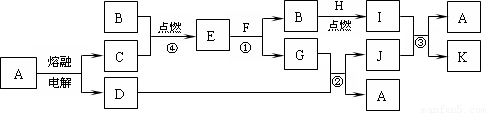
£Ø8·Ö£©ĻĀĶ¼ŹĒ֊ѧ»Æѧ֊³£¼ūĪļÖŹÖ®¼äµÄŅ»Š©·“Ó¦¹ŲĻµ£¬ĘäÖŠ²æ·Ö²śĪļĪ“Š“³ö”£³£ĪĀĻĀXŹĒ¹ĢĢ壬BŗĶGŹĒŅŗĢ壬ĘäÓą¾łĪŖĘųĢ壬FŹĒŗģ×ŲÉ«ĘųĢ唣øł¾ŻĻĀĶ¼¹ŲĻµĶʶĻ£ŗ
(1)»ÆѧŹ½£ŗX ___________”£
(2) Š“³öC”Ŗ”śEµÄ»Æѧ·“Ó¦·½³ĢŹ½______________________________________
(3) ĒėŠ“³öFÓėB·“Ӧɜ³ÉGµÄ»Æѧ·½³ĢŹ½£ŗ____________________£¬øĆ·“Ó¦¹ż³ĢÖŠŃõ»Æ²śĪļŗĶ»¹Ō²śĪļµÄĪļÖŹµÄĮæÖ®±ČĪŖ__________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012Ń§ÄźÉ½¶«ĮŁĒåČżÖŠµŚŅ»Ń§ĘŚøßŅ»ŌĀæ¼»Æ Ń§ ŹŌ ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø17·Ö£©ĻĀĶ¼ŹĒ֊ѧ»Æѧ֊³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµ£¬²æ·ÖĪļÖŹŗĶ·“Ó¦Ģõ¼žĀŌČ„”£
£Ø1£©µ„ÖŹBµÄ»ÆѧŹ½ŹĒ £¬µ„ÖŹFµÄ»ÆѧŹ½ŹĒ________ £¬Š“³öĪļÖŹAµÄŅ»ÖÖÓĆĶ¾ ”£
£Ø2£©Š“³öµ„ÖŹBÓėĒæ¼īČÜŅŗ·“ Ó¦µÄĄė×Ó·½³ĢŹ½ £¬
Ó¦µÄĄė×Ó·½³ĢŹ½ £¬
Š“³öÓɳĮµķJÉś³É HČÜŅŗµÄĄė×Ó·½³ĢŹ½ £¬
£Ø3£©ČÜŅŗEÖŠ¼ÓČė°±Ė®Ź±£¬ĻČÉś³É°×É«³ĮµķL£¬Š“³öÉś³ÉLµÄĄė×Ó·½³ĢŹ½ £¬
°×É«³ĮµķL»įŃøĖŁ±äĪŖ É«£¬×īÖÕ±äĪŖŗģŗÖÉ«³ĮµķG£¬Š“³öL±äĪŖGµÄ»Æѧ·“Ó¦·½³ĢŹ½ ”£
£Ø4£©ČÜŅŗIÖŠĖłŗ¬½šŹōĄė×ÓŹĒ_____________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016½ģĖÄ“ØŹ”øßŅ»ÉĻѧʌµŚČż“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ĻĀĶ¼ŹĒ֊ѧ»Æѧ֊³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµ£¬²æ·ÖĪļÖŹŗĶ·“Ó¦Ģõ¼žĀŌČ„”£

£Ø1£©G¼ÓČČæÉ·Ö½āÉś³ÉA£¬AµÄ»ÆѧŹ½ĪŖ________,Š“³öĪļÖŹAµÄŅ»ÖÖÓĆĶ¾ ”£
£Ø2£©Š“³öµ„ÖŹBÓėĒæ¼īČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½_______________________________________£¬Š“³öÓɳĮµķJÉś³É HČÜŅŗµÄĄė×Ó·½³ĢŹ½___________________________________________.”£
£Ø3£©ČÜŅŗEÖŠ¼ÓČė°±Ė®Ź±£¬ĻČÉś³É°×É«³ĮµķL£¬Š“³öÉś³ÉLµÄĄė×Ó·½³ĢŹ½_______________________£¬°×É«³ĮµķL»įŃøĖŁ±äĪŖ É«£¬×īÖÕ±äĪŖŗģŗÖÉ«³ĮµķG£¬Š“³öL±äĪŖGµÄ»Æѧ·“Ó¦·½³ĢŹ½ ”£
£Ø4£©ČÜŅŗIÖŠĖłŗ¬½šŹōĄė×ÓŹĒ_____________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com