
| ���¡���ѹ |
| ���� |
| 716.8L |
| 22.4mol?L-1 |
| ���¸�ѹ |
| ���� |
| ���¸�ѹ |
| ���� |
| 4mol |
| 16mol |
| 12mol |
| 24mol |


 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
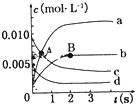 ��2L�ܱ������У�800��ʱ��Ӧ2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������У�800��ʱ��Ӧ2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����| ʱ�䣨t�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n��NO����mol�� | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Al-Mg�ڲ�ͬ�ĵ������Һ�зֱ�ԭ���A��B����ͼ��ʾ��
Al-Mg�ڲ�ͬ�ĵ������Һ�зֱ�ԭ���A��B����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
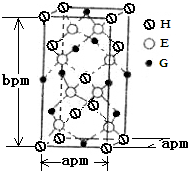 A��B��D��E��G��H����ǰ�����ڵ�Ԫ�أ�������ǰ�����ڵĻ�̬ԭ����A��δ�ɶԵ�����ࣻB��̬ԭ�ӵ�L���Ӳ��p�ܼ�����һ���չ����D �Ļ�̬ԭ�ӵ�2p�������1�����ӵ������������������ӵ����������෴��E�Ļ�̬ԭ��M����6���˶�״̬��ͬ�ĵ��ӣ� G�����ڱ���λ�ڵ�8�У�H��ԭ��������G��3��������Ϣ�ش��������⣺
A��B��D��E��G��H����ǰ�����ڵ�Ԫ�أ�������ǰ�����ڵĻ�̬ԭ����A��δ�ɶԵ�����ࣻB��̬ԭ�ӵ�L���Ӳ��p�ܼ�����һ���չ����D �Ļ�̬ԭ�ӵ�2p�������1�����ӵ������������������ӵ����������෴��E�Ļ�̬ԭ��M����6���˶�״̬��ͬ�ĵ��ӣ� G�����ڱ���λ�ڵ�8�У�H��ԭ��������G��3��������Ϣ�ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
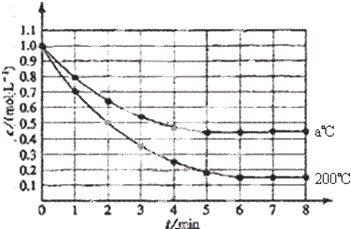
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com