
ij�о���ѧϰС���ͬѧ��ʵ���Ҷ�Cl2ʹ��ɫ������ɫ�Ļ���������̽����
��ͬѧ���������ͼ��ʾ��ʵ��װ�ò�����ʵ�飺
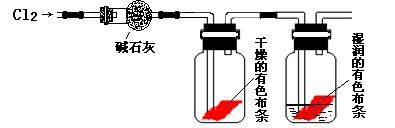
��1�� ��ͬѧʵ���û�еõ�Ԥ�ڵ�ʵ�������������������ʵ��ʧ�ܵ�ԭ��
��2����ͬѧ��ʵ��ʧ�ܺ����Ƶ�ʵ��װ�ý����˸Ľ��������½�����ʵ�飬����õ���Ԥ�ڵ�ʵ����������Ϊ��Ԥ�ڵ�ʵ�������� ���ɴ˵ó�Cl2ʹ��ɫ������ɫ�Ļ����� ![]() ��
��
��3����ͬѧ��Ϊ����Ƶ�ʵ��װ�ü�ʹ�Ľ���ȱ��һװ�á�ȱ�ٵ�װ���з��������ӷ�Ӧ����ʽΪ
 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ������Ƥ�����н�ǿ����ʴ�ԣ��������г��õĽ�������֮һ����п��������Ƥ�ı����㣬���Ĥ�ĺ�ȼ����ȶ�Ҳ�����ж϶Ʋ���������Ҫָ�꣮ij�о���ѧϰС��Ϊ�˲ⶨ��п��Ƥ�ĺ�ȣ�����������ʵ�鷽����
������Ƥ�����н�ǿ����ʴ�ԣ��������г��õĽ�������֮һ����п��������Ƥ�ı����㣬���Ĥ�ĺ�ȼ����ȶ�Ҳ�����ж϶Ʋ���������Ҫָ�꣮ij�о���ѧϰС��Ϊ�˲ⶨ��п��Ƥ�ĺ�ȣ�����������ʵ�鷽����
| ||
| 2S��7.14 |
| ||
| 2S��7.14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 Al��OH��3+3H+��Cu2++2H2O
Al��OH��3+3H+��Cu2++2H2O Cu��OH��2+2H+��2Al+6H+=2Al3++3H2��
Cu��OH��2+2H+��2Al+6H+=2Al3++3H2�� Al��OH��3+3H+��Cu2++2H2O
Al��OH��3+3H+��Cu2++2H2O Cu��OH��2+2H+��2Al+6H+=2Al3++3H2��
Cu��OH��2+2H+��2Al+6H+=2Al3++3H2��| ѡ�� | ���缫 | ����� | ������Ӧ | ������Ӧ |
| A | ���� | NaOH | Al-3e-=Al3+ | 2H2O+2e-=2OH-+H2�� |
| B | ���� | ϡ���� | 2Al-6e-=2Al3+ | 6H++6e-=3H2�� |
| C | ���� | Ũ���� | Cu-2e--=Cu2+ | 2NO3-+4H+-4e-=2NO2��+2H2O |
| D | ���� | ϡ���� | Cu-2e-=Cu2+ | 2NO3-+8H+=2NO��+4H2O+6e- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | �ܽ�ȣ�g/100ˮ�� | ���� | �ܽ�ȣ�g/100ˮ�� |
| Ca��OH��2 | 0.173 | Ba��OH��2 | 3.89 |
| CaCO3 | 0.0013 | BaSO3 | 0.016 |
| Ca��HCO3��2 | 16.60 |
| ʵ�鷽�� | ���� |
| 1��ȡ������Һ���Թ��У�����ϡ���ᣬ���ȣ� ��ʪ�����ɫʯ����ֽ�������ɵ����壮 |
|
| 2��ȡ������Һ���Թ��У����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�˴Ӻ��� FeCl3��FeC12��CuC12 �ķ�Һ�л���Cu��ij�о���ѧϰС���ͬ����������ַ�����
����1 �����Һ�м�����������ۣ���ַ�Ӧ���ˡ� �����������м������������ᣬ��ַ����ٹ��˼��õ�ͭ��
����1���漰�����������ӵ���������ǿ������˳��Ϊ��_____________________���˲���ʱ����Ҫ�õ��IJ��������ǣ�______________________
����2���ڷ�Һ�м������������������Һ��pH=1����ͭ��ʯī���缫���е�⡣���۲쵽���������������ݲ���ʱ����ֹͣ��⣬��ʱҪ���յ�Cu��ȫ��������
����2��ͭ��______�����������ĵ缫��ӦΪ(���ж���缫��Ӧ���밴�շ�Ӧ�������Ⱥ�˳��ȫ��д��)_______________________________________________________ ��һ�缫�������ĵ缫��ӦΪ_____________________________ ����2�ڵ缫��ֱ�ӻ���ͭ�������ϱȷ���1��㣬������2Ҳ�в���֮������Ҫ����Ϊ�� ______________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

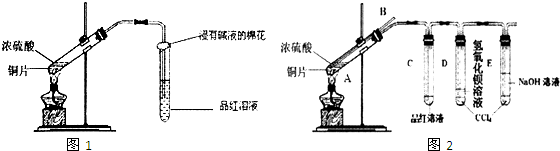
(1)������װ�������Եļ��鷽����_________________________________________________��
(2)�μ������ŨNaOH��Һ��˳����______________________________________________��
(3)ʵ������г���ͷ�ι�����Ƶ���ֽƬ�ı仯������______________________________��
(4)Aƿ��ʢ�ŵ��Լ���__________________________________________________________��
(5)Bƿ��������_________________________________________________________________��
(6)��ͬѧ˵����ʵ�鲻����ȫ����ʵ��Ŀ�ģ���������______________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com