
 Fe(OH)3+3H+
Fe(OH)3+3H+ 2NH3(g)
2NH3(g)  =-92.0kJ/mol
=-92.0kJ/mol Fe(OH)3+3H+��
Fe(OH)3+3H+�� 2NH3(g)
2NH3(g)  =-92.0kJ/mol��
=-92.0kJ/mol��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
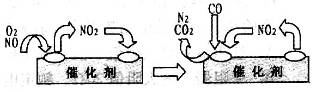 ��
��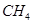 ����ԭ
����ԭ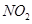 �ķ���Ҳ�������������������Ⱦ�����磺
�ķ���Ҳ�������������������Ⱦ�����磺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
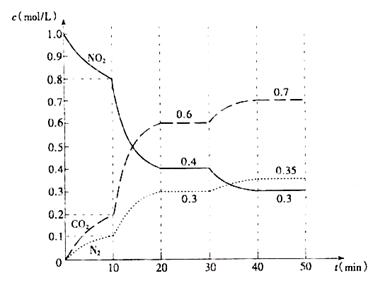
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
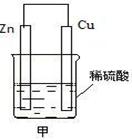
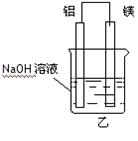
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��12��H3+5��H2-2��H1 | B��2��H1-5��H2-12��H3 |
| C��12��H3-5��H2 -2��H1 | D����H1-5��H2-12��H3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3��g����H����92kJ/mol����ʾ������2 mol NH3ʱ�ų�92.2KJ���ȣ����йؼ��ܣ�N��N��945.6kJ/mol ��N-H��391.0kJ/mol����H��H����Ϊ KJ/mol
2NH3��g����H����92kJ/mol����ʾ������2 mol NH3ʱ�ų�92.2KJ���ȣ����йؼ��ܣ�N��N��945.6kJ/mol ��N-H��391.0kJ/mol����H��H����Ϊ KJ/mol�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��+184.6kJ��mol-1 | B���D92.3kJ��mol-1 |
| C��+92.3kJ | D��+92.3kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 4CO2(g) + 2H2O(l) + 2600 kJ
4CO2(g) + 2H2O(l) + 2600 kJ 12CO2(g) + 6H2O(l) + 6590 kJ
12CO2(g) + 6H2O(l) + 6590 kJ | A��2mol C2H2(g) ��ȫȼ��������̬ˮʱ���ȴ���2600kJ |
| B��2mol C6H6(l) ��ȫȼ������Һ̬ˮʱ����С��6590kJ |
| C����ͬ�����£���������C2H2(g)��C6H6(g)��ȫȼ�գ�C6H6(g)���ȸ��� |
| D��C2H2(g) ��������C6H6(g) �Ĺ������ڷ��ȷ�Ӧ |
�鿴�𰸺ͽ���>>
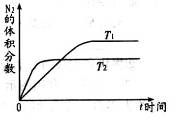
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

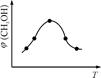 ��
�� 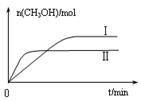
 CH3OH(g) +H2O(g) ��H
CH3OH(g) +H2O(g) ��H| �� �� | �� | �� |
| ��Ӧ�� Ͷ���� | 1molCO2 3molH2 | a molCO2��b molH2�� c molCH3OH(g)��c molH2O(g) |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com