
£Ø12·Ö£©ÓŠŅ»ÖÖ¹ć·ŗÓĆÓŚĘū³µ”¢¼Ņµē²śĘ·ÉĻµÄøß·Ö×ÓĶæĮĻ£¬ŹĒ°“ĻĀĮŠĮ÷³ĢĶ¼Éś²śµÄ”£Į÷³ĢĶ¼ÖŠ£ŗAŗĶM£ØC3H4O£©¶¼æÉ·¢ÉśŅų¾µ·“Ó¦£¬MŗĶNµÄ·Ö×ÓÖŠĢ¼Ō×ÓŹżĻąĶ¬£¬AµÄĢž»łÉĻµÄŅ»ĀČ“śĪļÓŠ3ÖÖ”£

£Ø1£©Š“³öĻĀŹöĪļÖŹµÄ½į¹¹¼ņŹ½£ŗM £¬D
£Ø2£©Š“³ö·ūŗĻĻĀĮŠĢõ¼žµÄĪļÖŹCµÄĶ¬·ÖŅģ¹¹Ģå £ØŠ“1øö¼“æÉ£©
¢ŁÓöŹÆČļŹŌŅŗ±äŗģ£»¢ŚøĆĪļÖŹµÄŗĖ“Ź²ÕńĒāĘ×ÉĻ¹²ÓŠ5øö·å£»¢Ū²»ÄÜŹ¹äåĖ®ĶŹÉ«
£Ø3£©N£«B”śCµÄ»Æѧ·½³ĢŹ½ĪŖ
£Ø4£©·“Ó¦ĄąŠĶ£ŗXĪŖ £¬YĪŖ
 Č«ÓÅ漵䵄ŌŖ¼ģ²ā¾ķ¼°¹éĄą×Üø“Ļ°ĻµĮŠ“š°ø
Č«ÓÅ漵䵄ŌŖ¼ģ²ā¾ķ¼°¹éĄą×Üø“Ļ°ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ÅØH2SO4 |
| ”÷ |
| ÅØH2SO4 |
| ”÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ÅØĮņĖį |
| ”÷ |
| ÅØĮņĖį |
| ”÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
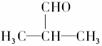
ŹŌŠ“³ö£ŗ
(1)ĪļÖŹµÄ½į¹¹¼ņŹ½£ŗA£ŗ_______________£¬M£ŗ_______________£»ĪļÖŹAµÄĶ¬Ąą±šµÄĶ¬·ÖŅģ¹¹Ģå£ŗ_____________________________________________”£
(2)N+B”śDµÄ»Æѧ·½³ĢŹ½£ŗ_____________________________________________”£
(3)·“Ó¦ĄąŠĶ£ŗX_______________£¬Y_______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğøŹĖąŗÓĪ÷ĪåŹŠøßČżµŚŅ»“ĪĮŖŗĻæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ÓŠŅ»ÖÖ¹ć·ŗÓĆÓŚĘū³µ”¢¼Ņµē²śĘ·ÉĻµÄøß·Ö×ÓĶæĮĻ£¬°“ĻĀĮŠĮ÷³ĢĶ¼Éś²ś”£Į÷³ĢĶ¼ÖŠM(C3H4O)ŗĶA¶¼æÉŅŌ·¢ÉśŅų¾µ·“Ó¦£¬NŗĶMµÄ·Ö×ÓÖŠĢ¼Ō×ÓŹżĻąµČ£¬AµÄĢž»łÉĻŅ»ĀČČ”“śĪ»ÖĆÓŠČżÖÖ”£

ĢīŠ“ĻĀĮŠæÕ°×£ŗ
¢ÅĪļÖŹµÄ½į¹¹¼ņŹ½£ŗA_________£¬øß·Ö×ÓĶæĮĻ___________________”£
¢ĘŠ“³öĻĀĮŠ×Ŗ»ÆµÄ·“Ó¦ĄąŠĶ£ŗA”śB_____£¬B”śD______”£
¢ĒŠ“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
A·¢ÉśŅų¾µ·“Ó¦£ŗ_______________________________________________£¬
BÓėN·“Ӧɜ³ÉD£ŗ_______________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010½ģŌĘÄĻŹ”øßČżøßæ¼³å“Ģ¾ķ£ØĪ壩ĄķæĘ×ŪŗĻ»ÆѧŹŌĢā ĢāŠĶ£ŗĢīæÕĢā
£Ø15·Ö£©ÓŠŅ»ÖÖ¹ć·ŗÓĆÓŚĘū³µ”¢¼Ņµē²śĘ·ÉĻµÄøß·Ö×ÓĶæĮĻ£¬ŹĒ°“ĻĀĮŠĮ÷³ĢĶ¼Éś²śµÄ”£Ķ¼ÖŠMŗĶA¶¼æÉ·¢ÉśŅų¾µ·“Ó¦£¬NŗĶMµÄ·Ö×ÓÖŠĢ¼Ō×ÓŹżĻąµČ£¬AµÄĢž»łÉĻµÄŅ»ĀČČ”“śĪ»ÖĆÓŠČżÖÖ”£

ŹŌŠ“³ö£ŗ
£Ø1£©ĪļÖŹµÄ½į¹¹¼ņŹ½£ŗA___________£¬M___________”£
£Ø2£©·“Ó¦ĄąŠĶ£ŗX____________£¬Y___________”£
£Ø3£©ĪļÖŹAµÄĶ¬·ÖŅģ¹¹Ģå½į¹¹¼ņŹ½ĪŖ£ŗ___________________________”££ØŠ“³öĮ½øö¼“æÉ£©”£
£Ø4£©D”śøß·Ö×ÓĶæĮĻµÄ»Æѧ·½³ĢŹ½£ŗ___________________________”£
£Ø5£©N+B”śDµÄ»Æѧ·½³ĢŹ½£ŗ___________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com