
���ǵ����ϼ�Ϊ�ḻ��Ԫ�ء�![]()
![]()
![]()
![]()
![]()
![]()
![]()
( 1 ) Li3N �����е��� N-3���ڣ���̬N-3�ĵ����Ų�ʽΪ�ߡ�
( 2 ) ![]() �ļ���Ϊ 942 kJ��mol-1 , N��N �����ļ���Ϊ 247kJ��mol-1������˵��N2 ��
�ļ���Ϊ 942 kJ��mol-1 , N��N �����ļ���Ϊ 247kJ��mol-1������˵��N2 ��
�� ���ȣ��ȶ������ ���� ������
( 3 ) ( CH3 )3 NH+ ��AlCl4-���γ�����Һ�塣����Һ����������������ɣ��۵���� 100 �� ����ӷ���һ����л��ܼ��ߣ����С�� ) ���������ߣ�����ţ���
a ����ȼ��
b ������ɫ���ܼ�
c �����ϲ���
d �����Ȳ���
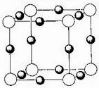
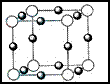 ( 4 ) x+�����е������ó��� K �� L �� M �������Ӳ㣬���� N3-�γɵľ���ṹ��ͼ��ʾ�� X��Ԫ�ط����ǣߣ���ͬһ��N3-������ x+���У߸���
( 4 ) x+�����е������ó��� K �� L �� M �������Ӳ㣬���� N3-�γɵľ���ṹ��ͼ��ʾ�� X��Ԫ�ط����ǣߣ���ͬһ��N3-������ x+���У߸���
�𰸣�32 ( l ) 1s22s22p6 ( 2 )�� �� ( 3 ��С b ( 4 ) Cu 6![]()
![]()
![]()
![]()
![]()
![]()
![]()
������Ҫ�������ʽṹ��ԭ������Ӧ�ã����ѡ�
�Ż�̬N3�����������Ŀ��10�����ݺ�������Ų������ƶϣ������Ų�ʽΪ��1s22s22p6��
�Ƹ���������ݿ�֪��N��N�������Ҽ��ļ���Ϊ247kJ/mol��N��N�ļ���Ϊ942kJ/mol����
�а���һ���Ҽ��������м�����м��ļ����ǣ�942-247��/2=347.5kJ/mol������Խ��ѧ��Խ�ȶ��������ݿ����жϦм��ȦҼ��ȶ���
���л��ܼ����ǹ��ۻ����������Һ����������������ɣ���������Ӧ�����Ӿ������ƣ��ӷ���С�ڹ��ۻ�����е����ԡ��������Һ���в����������䲻��ȼ�����������һ�㲻�������ϲ��ϣ�ѡb��
����֪X+���е������ó���K��2������L��8������M�㣨18�����������������Ϊ��28����Xԭ�ӵĺ��������Ŀ��29���ʺ˵������29����ͭԪ�ص�ԭ�ӣ��������ͼʾ����ṹ��֪��N3��λ��С������Ķ����ϣ�Cu+λ��������ÿ������е��ϣ�����ͬһ��N3��������Cu+��6����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��
��
 ��
��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧ�� | N-N | N=N | N��N | N-H | H-H |
| ����/kJ?mol-1 | 159 | 418 | 946 | 391 | 436 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com