
��ͼΪ������ļ���������
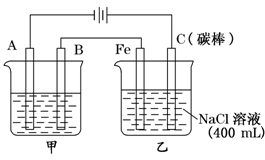

�Իش�
��1���׳���Ϊ�õ��ԭ������ͭ��װ�ã���B�� ���������� ��
A�缫��ӦΪ ��
��2���ҳ�������������ʯ����Һ�����һ��ʱ���Fe�������� ɫ��
��3�����׳�Ϊ��⾫��ͭ����������6.4g�����ҳ��������ų��������ڱ�״���µ����Ϊ ������ʱ�ҳ�ʣ��Һ��Ϊ400 mL�������õ���Һ�����ʵ���Ũ��Ϊ ��
��4���ھ���ͭ�Ĺ����У��������Һ��c(Fe2+)��c(Zn2+)���������Ӱ���һ����⡣�������ʵ��ܶȻ�������KSP����
| ���� | Fe(OH)2 | Fe(OH)3 | Zn(OH)2 | Cu(OH)2 |
| KSP | 8.0��10��16 | 4.0��10��38 | 3.0��10��17 | 2.2��10��20 |
���ڵ��Һ��pH�dz�ȥ�������ӵij��÷����������ϱ����ܶȻ������жϣ����е����ʵ���Ũ�ȵ�Fe2+��Zn2+��Fe3+��Cu2+����Һ����pH�������ȳ���������������______________��
һ�ַ������ȼ��������H2O2���ٵ���pH��4���ҡ�����H2O2������Ӧ�����ӷ���ʽΪ__________________________________________________________��
 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼΪ������ļ����������أ���ش�
��ͼΪ������ļ����������أ���ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼΪ������ļ����������أ���ش�
��ͼΪ������ļ����������أ���ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼΪ������ļ����������أ���ش�
��ͼΪ������ļ����������أ���ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�Ƹ���ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��ͼΪ������ļ���������
�Իش�
��1���׳���Ϊ�õ��ԭ������ͭ��װ�ã���A�� ���������� ��
�缫��ӦΪ ��
��2���ҳ���������������̪��Һ�����һ��ʱ���Fe�������� ɫ��
��3�����׳�Ϊ��⾫��ͭ����������12.8g�����ҳ��������ų��������ڱ�״���µ����Ϊ
������ʱ�ҳ�ʣ��Һ��Ϊ400 mL�������õ���Һ�����ʵ���Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��20�֣���ͼΪ������ļ����������أ���ش�

��1�����׳�Ϊ��⾫����ͭ������п��������Ͳ��������ʣ����ʷ����ĵ缫��Ӧ����д����װ�ã����Һѡ��CuSO4��Һ����
��A�缫�����ķ�Ӧ����Ϊ �� B�缫��Fe�缫���ӵ������� �� ����B��Fe����
��B�缫�IJ�����____________���缫��ӦʽΪ___________ _______��
CuSO4��Һ��Ũ�� ���� �����䡱���������ӡ������м��١���
(2)ʵ�鿪ʼʱ�������ҳ����ߵ缫����ͬʱ�����뼸�η�̪��Һ����
��д�����NaCl��Һ�ܵĻ�ѧ����ʽ
��
����Fe�������۲쵽��������____ ________������̼���缫��Ӧ����ķ����� _��
(3)���ײ۵��ҺΪCuSO4��ʵ���з�����������12.8g�����Ҳ������ų������ڱ�״���µ����Ϊ_____ ____L�����Ҳ�ʣ��Һ��Ϊ400mL�������õ���Һ�����ʵ���Ũ��Ϊ_______ ___ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com