
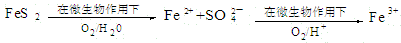


 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ɫ��Ӧ֪ʶ�йأ��������н������仯��������ʱ���������������ɫ�� |
| B����̼������һ��ʱ�е�������������������������ŷţ�CO2��CH4���dz������������塣 |
| C����ͥȼ��й©Ӧ����ͨ���Ա��ⱬը������ |
| D����ʯ�������Mg3(Si4O10)(OH)2����������������ʽ��ʾΪ4MgO��4SiO2��H2O�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ѧ����ʽΪ ��
ѧ����ʽΪ ��


 ����һ��ˮ���Ե�ά���أ�ȱ��VC��ʹ�˻��� ������ȡһƬ����ˮ�����������ϵμӵ�����Һ�͵�ˮ�������� ��˵������ˮ���к���VC����������VC���� ����
����һ��ˮ���Ե�ά���أ�ȱ��VC��ʹ�˻��� ������ȡһƬ����ˮ�����������ϵμӵ�����Һ�͵�ˮ�������� ��˵������ˮ���к���VC����������VC���� ���� ����������ԭ�������ʡ�
����������ԭ�������ʡ� ��������Ϊ��˾ƥ�֣��dz���ҩ������ˮ�ܻ�����ˮ������ˮ���ᣬ��ˮ����Ľṹ��ʽ�ɱ�ʾΪ
��������Ϊ��˾ƥ�֣��dz���ҩ������ˮ�ܻ�����ˮ������ˮ���ᣬ��ˮ����Ľṹ��ʽ�ɱ�ʾΪ  ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ж���
| A��NaHCO3 | B��CaCO3 | C��Na2CO3 | D��NH4HCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
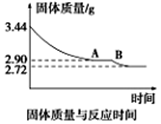
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
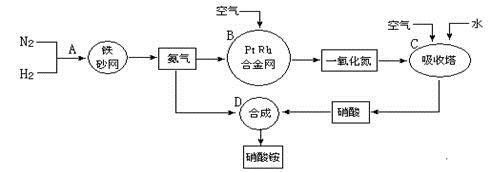

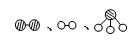 �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں͢۵ĺ���ֱ��� �� ��
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں͢۵ĺ���ֱ��� �� ��| | ע������ | ���� |
| �� | | |
| �� | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��NH3 | B��CH4 | C��CO2 | D��H2 |
�鿴�𰸺ͽ���>>
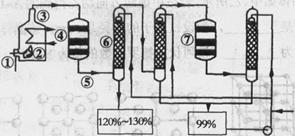
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ���� | ����CO(NH2)2 | ̼�NH4HCO3 | ���Ca3(PO4)2 |
| �۸�(Ԫ/��) | 1 200 | 350 | 150 |
| ���� | �������Ca(H2PO4)2 | �Ȼ��� | ����� |
| �۸�(Ԫ/��) | 250 | 650 | 800 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��KH2PO4��NH4Cl | B��NH4Cl��NH4NO3 |
| C��KNO3��KH2PO4 | D��NH4Cl��KNO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com