

 ·½³ĢŹ½±ķŹ¾ĘäŌŅņ£ŗ
·½³ĢŹ½±ķŹ¾ĘäŌŅņ£ŗ ”””” (ø÷1·Ö)
”””” (ø÷1·Ö) 2SO3(2·Ö)
2SO3(2·Ö) NH3”¤ H2O
NH3”¤ H2O NH4++OH- (2·Ö)
NH4++OH- (2·Ö)


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŌŖĖŲŠŌÖŹĖęŌ×ÓŠņŹżµÄµŻŌö³ŹÖÜĘŚŠŌ±ä»ÆµÄøł±¾ŌŅņŹĒŌŖĖŲµÄ»ÆŗĻ¼Ū³ŹÖÜĘŚŠŌ±ä»Æ |
| B£®ŗĖĶāµē×Óøł¾ŻĘäÄÜĮæµÄ²ī±š“¦ÓŚ²»Ķ¬µÄµē×Ó²ć£¬ÄÜĮæŌ½øߥėŗĖŌ½½ü |
| C£®ĖłÓŠŌ×ÓµÄŌ×ÓŗĖ¶¼ŹĒÓÉÖŹ×ÓŗĶÖŠ×Ó¹¹³ÉµÄ |
| D£®ŌŚŌŖĖŲÖÜĘŚ±ķÖŠ¾ßÓŠĻąĶ¬µÄµē×Ó²ćŹżµÄÖ÷×åŌŖĖŲ£¬Ėł“¦µÄÖÜĘŚŠņŹżĻąĶ¬ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| ŌŖĖŲ | Ļą¹ŲŠÅĻ¢ |
| X | XŌ×ÓŗĖĶā×īĶā²ćµē×ÓŹżŹĒ“ĪĶā²ćµÄ2±¶ |
| Y | YµÄĘųĢ¬Ēā»ÆĪļµÄĖ®ČÜŅŗĻŌČõ¼īŠŌ |
| Z | ZŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄ½šŹōŌŖĖŲ |
| W | ³£ĪĀ³£Ń¹ĻĀ£¬WµÄµ„ÖŹŹĒµ»ĘÉ«¹ĢĢå |
| Q | ”” |
 (1)ŌŖĖŲQŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆ______________________________£®
(1)ŌŖĖŲQŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆ______________________________£®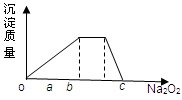
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
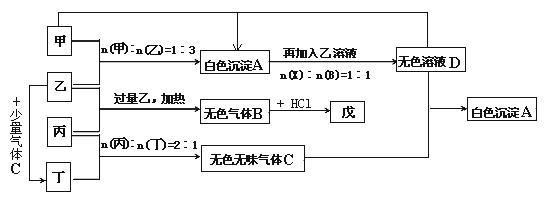
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 £¬µŚ________×壻
£¬µŚ________×壻 ) Š“³öĶÓėĻ”HBO3ČÜŅŗ·“Ó¦µÄĄė×Ó·“Ó¦·½³ĢŹ½
) Š“³öĶÓėĻ”HBO3ČÜŅŗ·“Ó¦µÄĄė×Ó·“Ó¦·½³ĢŹ½ ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
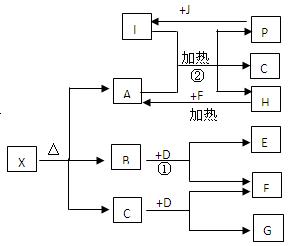
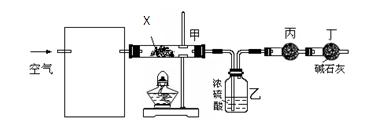
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 Ć¾£¬Ö÷ŅŖ¹ż³ĢČēĻĀ£ŗ
Ć¾£¬Ö÷ŅŖ¹ż³ĢČēĻĀ£ŗ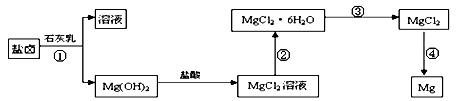
 »Ų“šĻĀĮŠĪŹĢā£ŗ
»Ų“šĻĀĮŠĪŹĢā£ŗ ”£
ӣ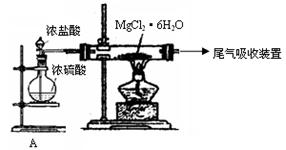
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com