
CO2��CO�ǹ�ҵ�ŷŵĶԻ�������Ӱ��ķ�����
��1����CO2��NH3Ϊԭ�Ϻϳɻ������ص���Ҫ��Ӧ���£�
��2NH3(g)��CO2(g)��NH2CO2NH4(s)����H����159.47 kJ��mol��1
��NH2CO2NH4(s)��CO(NH2)2(s)��H2O(g)����H��a kJ��mol��1
��2NH3(g)��CO2(g)��CO(NH2)2(s)��H2O(g)����H����86.98 kJ��mol��1
��aΪ ��
��2����ѧ��������ù�ҵ�����е�CO2��ȡ�״���CO2+3H2CH3OH+H2O���Ƶõ�CH3OH������ȼ�ϵ�ص�ȼ�ϡ�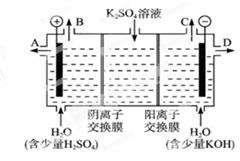
����KOH�����У������ĵ缫��ӦʽΪ_________________________________��
�������ʵ�KOH�����õ��K2SO4��Һ�ķ����Ƶá���KOH��_______���ڵõ�,�����ĵ缫��Ӧʽ�ǣ�_____________________________________��
��3������CO��H2��Ӧ�ɺϳ�CH3OCH3��
��֪��3H2(g) + 3CO(g)  CH3OCH3(g) + CO2(g)����H��-247kJ/mol
CH3OCH3(g) + CO2(g)����H��-247kJ/mol
��һ�������µ��ܱ������У��÷�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� .
| A�����¸�ѹ�� | B����������� | C�����������뺤���� | D������CO��Ũ�ȣ�E������������� |
 CH3OCH3(g) + H2O(g)����ij�¶��£���1L�ܱ������м���CH3OH ����Ӧ��10����ʱ�ﵽƽ�⣬��ʱ��ø���ֵ�Ũ�����£�
CH3OCH3(g) + H2O(g)����ij�¶��£���1L�ܱ������м���CH3OH ����Ӧ��10����ʱ�ﵽƽ�⣬��ʱ��ø���ֵ�Ũ�����£�| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol��L��1�� | 0.01 | 0.2 | 0.2 |
��1��+72.49kJ��mol��1 ��2�֣�
��2����CH3OH -6e-+8OH-=CO32-+6H2O��2�֣�
��D ��2�֣�4OH- + 4e- = 2H2O + O2�� ��2�֣�
��3��AE ��2�֣�
��4����0.04 mol��L-1��min-1��2�֣��� 400��2�֣� �� �� ��2��
���������������1�������Ȼ�ѧ����ʽ��˹���ɢ�+��=�ۣ��õ�-159.47KJ/mol+a=-86.98KJ/mol��a=+72.49KJ/mol��
��2���ټ״���ȼ���Ƴɵ�ȼ�ϵ�����ɵ�CO2������Һ��������K2CO3���ܷ�ӦʽΪ��2CH3OH+4KOH+3O2=2K2CO3+6H2O,���Լ״��ڸ���ʧȥ���ӣ������ĵ缫��ӦʽΪ��2CH3OH-12e- +16OH- =2CO32- +12H2O��������Ϊ��3O2+12e-+6H2O=12OH-
�ڵ��K2SO4��Һʵ�ʾ��ǵ��ˮ������ˮ�����OH-ʧȥ���ӱ�������������������H+��SO42-ͨ�������ӽ���Ĥ������������A�ڳ��õ����ᣬ�����缫��ӦʽΪ��4OH- + 4e- = 2H2O + O2����������ˮ�����H+�õ����ӱ���ԭ�����Ի��д���OH-���ɣ�K+ͨ�������ӽ���Ĥ������������D�ڳ��õ�KOH��������ӦʽΪ:4H++ 4e- =2H2����
��3���ɷ�Ӧ��֪����ӦΪ���ȡ�ǰ������ϵ�����ٵķ�Ӧ����������ѹǿ�������¶�����������Ӧ���CO��ת���ʣ�����A���У��������ֻ�ܼӿ췴Ӧ���ʣ����ܸı�ƽ�⣬B�����У����������뺤�����ı���μӷ�Ӧ�����Ũ�ȣ�����ı�ƽ�⣬���C������COת���ʣ�����CO��Ũ����ʹƽ�������ƶ�������CO��ת���ʷ������ͣ�E����������������ѣ�����ʹ��Ӧ�����ƶ������������CO��ת���ʣ�����E���У���ѡAE��
��4�����ݱ��������У�2CH3OH(g)  CH3OCH3(g) + H2O(g)
CH3OCH3(g) + H2O(g)
��ʼŨ�ȣ�mol/L���� 0.41 0 0
ת��Ũ�ȣ�mol/L���� 0.4 0.2 0.2
ƽ��Ũ�ȣ�mol/L���� 0.01 0.2 0.2
���Ԣ�0-10 min�ڷ�Ӧ����v(CH3OH) ��0.4 mol/L��10min=0.04 mol��L-1��min-1
�ڸ��¶��µ�ƽ�ⳣ��K=0.2��0.2��0.012 =400
����Ϊ��ƽ��ʱ����������0.01mol CH3OH��0.2mol CH3OCH3 ��0.2mol H2O�������º����������������ټ���ͬ����0.01mol CH3OH��0.2mol CH3OCH3��0.2mol H2O����ﵽ��Чƽ�⣬ƽ�ⲻ�ƶ�������ֻ����0.01mol CH3OH��0.2mol CH3OCH3 ���ܴﵽ��Чƽ�⣬ƽ��������Ӧ�����ƶ������Դ�ʱv��>v�� ��
���㣺���⿼����ǻ�ѧ��Ӧ���������绯ѧ��������ѧ��Ӧ������ƽ���֪ʶ���ۺϡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ʵ��Ļ�ѧ������ա�
��1��Na2CO3ˮ������ӷ���ʽ�� ��
��2��H2S���뷽��ʽ�� ��
��3��AlCl3ˮ������ӷ���ʽ�� ��
��4����25�桢101 kPa�£�l g������ȫȼ������CO2��Һ̬ˮʱ����55��6 kJ������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ�� ��
��5����������ȼ�ϵ�ص������缫����ʽ
������ ��
������ ��
��6��д��NaHCO3��Һ�е�����Ũ�ȹ�ϵ
c(H+)+c(Na+)= ��
c(Na+)= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Դ����Լ���ҷ�չ���̵�����֮һ���״��������ѵȱ���Ϊ2 1���͵���ɫ��Դ����ҵ��������Ȼ��Ϊ��Ҫԭ���������̼��ˮ������һ���������Ʊ��ϳ�����CO��H2�������Ƴɼ״��������ѡ�
��1����ҵ�ϣ����Է���ϳ����е����������ںϳɰ������ô����������ͭ
[Cu��NH3��2Ac]��Һ��Ac=CH3COO�����������պϳ����е�һ����̼���䷴��ԭ��Ϊ��
[Cu��NH3��2Ac]��aq��+CO+NH3 [Cu��NH3��3]Ac?CO��aq������H��0��
[Cu��NH3��3]Ac?CO��aq������H��0��
��ѹ�£�������һ����̼����Һ�������»��[Cu��NH3��2]AC��Һ�Ĵ�ʩ�� ��
��2����ҵ��һ������������ַ�Ӧ�ϳɼ״���
��Ӧa��CO2��g��+3H2��g�� CH3OH��g��+H2O��g�� ��H����49.0kJ/mol
CH3OH��g��+H2O��g�� ��H����49.0kJ/mol
��Ӧb��CO��g��+2H2��g�� CH3OH��g�� ��H<0
CH3OH��g�� ��H<0
�ٶ��ڷ�Ӧa��ij�¶��£���4.0 mol CO2��g����12.0 mol H2��g�������ݻ�Ϊ2L���ܱ������У���Ӧ����ƽ��ʱ����ü״��������������Ϊ30%������¶��·�Ӧ��ƽ�ⳣ��Ϊ ��
�ڶ��ڷ�Ӧb��ij�¶��£���1.0mol CO��g����2��0 mol H2��������̶��ݻ����ܱ������У���Ӧ����ƽ��ʱ���ı��¶Ⱥ�ѹǿ��ƽ����ϵ��CH3OH�����ʵ��������仯�����ͼ��ʾ���¶Ⱥ�ѹǿ�Ĺ�ϵ�ж���ȷ���� ��������ĸ���ţ�
A��p3>p2��T3>T2
B��p2>p4��T4>T2
C��p1>p3��T1>T3
D��p1>p4��T2>T3
��3��CO���Ժϳɶ����ѣ������ѿ�����Ϊȼ�ϵ�ص�ԭ�ϣ���ѧ��Ӧԭ��Ϊ��
CO��g��+4H2��g�� CH3OCH3��g��+H2O��g�� ��H<0
CH3OCH3��g��+H2O��g�� ��H<0
���ں����ܱ������ﰴ�����Ϊ1:4����һ����̼��������һ�������·�Ӧ�ﵽƽ��״̬�����ı䷴Ӧ��ijһ�����������б仯��˵��ƽ��һ��������Ӧ�����ƶ����� ��
A���淴Ӧ������������С
B������Ӧ������������С
C����Ӧ�������ٷֺ�����С
D����ѧƽ�ⳣ��Kֵ����
��д�������Ѽ���ȼ�ϵ�صĸ����缫��Ӧʽ ��
�ۼ�֪����缫��Ӧ�ĵ缫���ϵ�λ�����ų����ܵĴ�С��Ϊ�õ�صı����������ڶ����Ѽ���ȼ�ϵ�����Ҵ�����ȼ�ϵ�أ�����˵����ȷ���� ������ĸ��
A������ȼ�ϻ�Ϊͬ���칹�壬����ʽ��Ħ��������ͬ����������ͬ
B������ȼ���������ۼ���Ŀ��ͬ���ϼ�ʱ����������ͬ����������ͬ
C������ȼ���������ۼ����Ͳ�ͬ���ϼ�ʱ����������ͬ����������ͬ
��4����֪l g������������ȫȼ�������ȶ���������ų�������Ϊ31.63 kJ����д����ʾ������ȼ���ȵ��Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ǵ����Ϻ�����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á���ش��������⣺
��1����ͼ��1 mol NO2��1mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ ��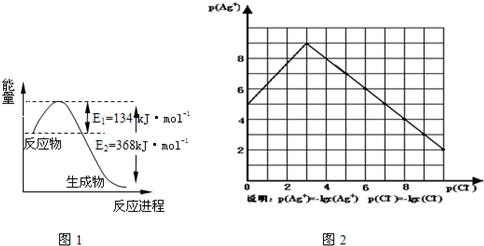
��2����0.5L���ܱ������У�һ�����ĵ����������������»�ѧ��Ӧ��N2(g)+3H2(g)  2NH3(g)��H<0�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���ұ���������������⣻
2NH3(g)��H<0�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���ұ���������������⣻
| t/�� | 200 | 300 | 400 |
| K | K1 | K2 | 0.5 |
 N2(g)+3H2(g)�Ļ�ѧƽ�ⳣ����ֵΪ �������NH3��N2��H2�����ʵ����ֱ�Ϊ3mol��2mol��1molʱ����÷�Ӧ��v��N2���� __ v��N2��������д��>������=����<��=��
N2(g)+3H2(g)�Ļ�ѧƽ�ⳣ����ֵΪ �������NH3��N2��H2�����ʵ����ֱ�Ϊ3mol��2mol��1molʱ����÷�Ӧ��v��N2���� __ v��N2��������д��>������=����<��=���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����һ�ֵ����Ϻ����ḻ��Ԫ�أ������仯������о���������������������Ҫ���塣
��1����ͼ��1 mol NO2��1 mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ ��
��2����֪��N2(g)+ O2(g)��2 NO(g) ��H����180 kJ ? mol-1
2NO(g)+2 CO(g)��N2(g) + 2 CO2(g) ��H����746 kJ ? mol-1
��ӦCO(g) + O2(g)��CO2(g)�� ��H�� kJ ? mol-1��
O2(g)��CO2(g)�� ��H�� kJ ? mol-1��
��3����һ�̶��ݻ�Ϊ2L���ܱ������ڼ���0.2 mol��N2��0.6 mol��H2����һ�������·������·�Ӧ�� N2(g)��3H2(g) 2NH3(g) ��H ��0�� ����5����ʱ�ﵽƽ�⣬��ʱ���NH3�����ʵ���Ϊ0.2 mol��ƽ��ʱH2��ת����Ϊ ��
2NH3(g) ��H ��0�� ����5����ʱ�ﵽƽ�⣬��ʱ���NH3�����ʵ���Ϊ0.2 mol��ƽ��ʱH2��ת����Ϊ ��
��4���ڹ̶�������ܱ������У�1.0��103 kPaʱ��������Ӧ N2(g)+3H2(g) 2NH3(g) ��H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3(g) ��H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
| T/K | 298 | 398 | 498 |
| ƽ�ⳣ��K | 51 | K1 | K2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����CO����ȼ�ϼ״���һ���¶Ⱥ��ݻ������·�����Ӧ��CO(g)+2H2(g) CH3OH(g)��ͼ1��ʾ��Ӧ�е������仯��ͼ2��ʾһ���¶��£������Ϊ1L���ܱ������м���2mol H2��һ������CO��CO��CH3OH(g)��Ũ����ʱ��仯��
CH3OH(g)��ͼ1��ʾ��Ӧ�е������仯��ͼ2��ʾһ���¶��£������Ϊ1L���ܱ������м���2mol H2��һ������CO��CO��CH3OH(g)��Ũ����ʱ��仯��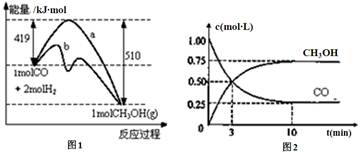
��ش��������⣺
��1���ڡ�ͼ1���У����� (�a����b��)��ʾʹ���˴�����û��ʹ�ô���ʱ���ڸ��¶Ⱥ�ѹǿ�����·�ӦCO(g)+2H2(g) CH3OH(g)�ġ�H= ��
CH3OH(g)�ġ�H= ��
��2������˵����ȷ����
A����ʼ�����CO�����ʵ���Ϊ1mol
B������CO��Ũ�ȣ�H2��ת���ʻ�����
C��������ѹǿ�㶨ʱ����Ӧ�ﵽƽ��״̬
��3���ӷ�Ӧ��ʼ������ƽ�⣬v(CO)= ���ﵽƽ��ʱ��c(H2)= �����¶���CO(g)+2H2(g)  CH3OH(g)�Ļ�ѧƽ�ⳣ��Ϊ ���ﵽƽ��������������������䣬���������ѹ��Ϊ0.5L����ƽ�� �ƶ� (�������������)��
CH3OH(g)�Ļ�ѧƽ�ⳣ��Ϊ ���ﵽƽ��������������������䣬���������ѹ��Ϊ0.5L����ƽ�� �ƶ� (�������������)��
��4����֪CH3OH(g)��3/2O2(g)��CO2(g)��2H2O(g)����H����193kJ/mol
��֪H2O(l)= H2O(g)����H����44 kJ/mol����д��32g��CH3OH(g)��ȫȼ������Һ̬ˮ���Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ú��ƶ�͡����������ҹ���Դ��չ���ٵ���״��������Դ��������ţ���չ��ú���������ҹ���Դ�ṹ�ĵ���������Ҫ���塣��ͼ��ú������ҵ��֮һ��
���ྻú�������о����������൱�ձ飬������Աͨ�������ú������¯�н�����������ˮ�����ķ���������������ֵ�ܸߵ�ú̿�ϳ���������Ҫ�ɷ���CO��H2��CO��H2����Ϊ��Դ�ͻ���ԭ�ϣ�Ӧ��ʮ�ֹ㷺��
��1����֪��C(s)+O2(g)=CO2(g) ��H1����393.5 kJ��mol�C1 ��
C(s)+H2O(g)=CO(g)+H2(g) ��H2����131.3 kJ��mol�C1 ��
��ӦCO(g)+H2(g) +O2(g)= H2O(g)+CO2(g)����H= _________kJ��mol�C1���ڱ�״���£�33.6 L��ú̿�ϳ���(��ȫ��ΪCO��H2)��������ȫ��Ӧ����CO2��H2O����Ӧ��ת��______mole����
��2����һ���ݵ��ܱ������У���CO��H2�ϳɼ״���CO(g)+2H2(g) CH3OH(g)
CH3OH(g)
������������˵��������Ӧ�Ѵﵽƽ��״̬����_______
a����ϵѹǿ���ֲ���
b���ܱ�������CO��H2��CH3OH(g)3�����干��
c��CH3OH��H2���ʵ���֮��Ϊ1:2
d��ÿ����1 mol CO��ͬʱ����2molH2
��CO��ƽ��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��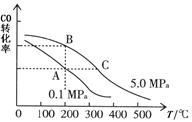
A��B�����ƽ�ⳣ��_____(�ǰ�ߡ��������ߡ���һ����)�ﵽA��C�����ƽ��״̬�����ʱ��tA tC(����ڡ�����С�ڡ����ڡ�)��
�ڲ��ı䷴Ӧ������������£�Ϊ���CO��ת���ʿɲ�ȡ�Ĵ�ʩ��_____________(������㼴��)��
��3�������¶�650���������ȼ�ϵ�أ�����ú̿��(CO��H2)������ȼ����������CO2�Ļ������Ϊ����ȼ������һ��������Li2CO3��Na2CO3���۵�����������ʣ��Խ�����(ȼ�ϼ�)Ϊ�����Ƴɵġ������ĵ缫��ӦʽΪ��CO + H2��4e�� + 2CO32��= 3CO2+H2O����õ�ص�������ӦʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������һ�ֽྻ����������Դ����������(��Ҫ�ɷ�Ϊ CO��CO2��H2��)��H2��ϣ����ϳɼ״��Ͷ����ѣ�CH3OCH3���������������ʵȣ��������������õķ���֮һ.
��1����֪̼��������Ӧ�ڲ�ͬ�¶���ƽ�ⳣ���Ķ���ֵ��lgK�����±�: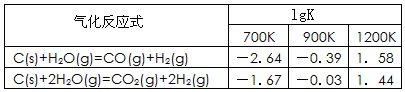
��Ӧ��CO(g)+H2O(g)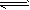 CO2(g)+H2(g)���÷�Ӧ�ġ�H________0��ѡ�����������������������������900Kʱ���÷�Ӧƽ�ⳣ���Ķ���ֵ��lgK��=_____________.
CO2(g)+H2(g)���÷�Ӧ�ġ�H________0��ѡ�����������������������������900Kʱ���÷�Ӧƽ�ⳣ���Ķ���ֵ��lgK��=_____________.
��2���״���һ����Ҫ����Դ�ͻ���ԭ�ϣ���ҵ�Ϻϳɼ״��ķ�ӦΪ��CO+2H2?CH3OH������֪��H2(g)��CO(g)��CH3OH(l)��ȼ���Ȧ�H�ֱ�Ϊ-285.8KJ/mol��-283.0KJ/mol��-726.5KJ/mol����CH3OH����ȫȼ������CO��Һ̬H2O���Ȼ�ѧ��Ӧ����ʽ .
��3����һ���¶ȡ�ѹǿ�ʹ������£���ҵ����CO��H2��Ӧ���ɶ����ѣ�ͬʱ����һ�ֲ������ѭ���������÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ�� ��
��4����ͼ��Ϊ��ɫ��Դ��������ȼ�ϵ�ء��Ĺ���ԭ��ʾ��ͼ��a�缫�Ϸ�����Ӧ�ĵ缫��ӦʽΪ .
��5��������ͼ��װ�õĵ�ԴΪ��4�����еĶ�����ȼ�ϵ�ء���ͨ��Դһ��ʱ��۲쵽װ���е������Һ��ɫ����ɫ��Ϊ��ɫ����������װ���е�Cu�缫Ӧ�������ȼ�ϵ���� �缫����a��b��������ͨ��ʱ������Ӧ���ܵ����ӷ�Ӧ����ʽΪ�� .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ��Ӧ��һ�������������ı仯����ѧ����ʵ�����������е�Ӧ�ý�Խ��Խ�㷺��
��1����������������ȡ��ҵԭ�����ᡣ��֪��
��CH3COOH(l)+2O2(g)=2CO2(g)+2H2O(l) ��H1 = ��870.3kJ��mo1
��C(s)+O2(g)=CO2(g) ��H 2= ��393.5kJ��mo1
��H2(g) + 1/2O2(g)=H2O(l) ��H 3= ��285.8kJ��mo1
������������Ϣ����������Ӧ�ķ�Ӧ�ȣ�
2C(s)+2H2(g)+O2(g)=CH3COOH(l) ��H= ��
��2����ѧ�ҿ����ʱ���ر����жϻ�ѧ��Ӧ���еķ������ж������Է����еĻ�ѧ��Ӧ�����������ȷ�Ӧ�ҷ�Ӧ��������Ϊ ������ĸ����
| A��2Na+2H2O=2NaOH+H2�� | B��3Fe+2O2=Fe3O4 |
| C��(NH4)2CO3=NH4HCO3+NH3�� | D��NaOH+HCl=NaCl+H2O |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com