
(10��)��֪A��B��C��D��E�ǻ�ѧ�г��������ʣ������£�E��һ����ɫ��ζ��Һ�壬����֮�������·�Ӧ��ϵ��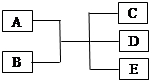
��1����A��һ�ֺ�ɫ���ʣ�B��һ�ֳ������ѻӷ����ᣬC��D�������壬��д���˷�Ӧ�Ļ�ѧ����ʽ ��
��2����A��һ���Ϻ�ɫ���ʣ�B��һ�ֳ����Ļӷ����ᣬ��Ӧʱ���ɵ�C����ɫ���壬��Ӧ�����ӷ���ʽ�� ��B�ڷ�Ӧ�б��ֳ��������� �� ��
��3����ʵ����������A��B�ķ�Ӧ�Ʊ�����C��C��һ����ɫ���̼�����ζ���ܶȱȿ���С�����壬��д���˷�Ӧ�Ļ�ѧ����ʽ ��ʵ���Ҽ���C�ķ��� ��
��10�֣�
��1��C+2H2SO4��Ũ��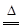 CO2��+ 2H2O + SO2����2�֣�
CO2��+ 2H2O + SO2����2�֣�
��2��2NO3��+ 3Cu + 8H�� = 2NO��+ 4H2O + 3 Cu 2�� ��2�֣����ԡ������ԣ�2�֣�
��3��2NH4Cl+ Ca(OH)2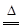 CaCl2 +2NH3��+2H2O��2�֣�����ʪ��ĺ�ɫʯ����ֽ�������壬����ֽ��������֤��������Ϊ������������պ��Ũ����IJ��������������壬���а������ɣ���֤��������Ϊ����������2�֣�
CaCl2 +2NH3��+2H2O��2�֣�����ʪ��ĺ�ɫʯ����ֽ�������壬����ֽ��������֤��������Ϊ������������պ��Ũ����IJ��������������壬���а������ɣ���֤��������Ϊ����������2�֣�
���������������1����A��һ�ֺ�ɫ���ʣ�B��һ�ֳ������ѻӷ����ᣬC��D�������壬��A��̼��B�����ᣬ�˷�Ӧ�Ļ�ѧ����ʽ��C+2H2SO4��Ũ��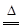 CO2��+ 2H2O + SO2����2����A��һ���Ϻ�ɫ���ʣ�B��һ�ֳ����Ļӷ����ᣬ��Ӧʱ���ɵ�C����ɫ���壬��A��ͭ��B�����ᣬ��Ӧ�����ӷ���ʽ��2NO3��+ 3Cu + 8H�� = 2NO��+ 4H2O + 3 Cu 2�� ��B�ڷ�Ӧ�б��ֳ������������ԡ������ԡ�
CO2��+ 2H2O + SO2����2����A��һ���Ϻ�ɫ���ʣ�B��һ�ֳ����Ļӷ����ᣬ��Ӧʱ���ɵ�C����ɫ���壬��A��ͭ��B�����ᣬ��Ӧ�����ӷ���ʽ��2NO3��+ 3Cu + 8H�� = 2NO��+ 4H2O + 3 Cu 2�� ��B�ڷ�Ӧ�б��ֳ������������ԡ������ԡ�
��3����ʵ����������A��B�ķ�Ӧ�Ʊ�����C��C��һ����ɫ���̼�����ζ���ܶȱȿ���С�����壬��C �ǰ������˷�Ӧ�Ļ�ѧ����2NH4Cl+ Ca(OH)2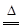 CaCl2 +2NH3��+2H2O��ʵ���Ҽ���C�ķ�����ʪ��ĺ�ɫʯ����ֽ�������壬����ֽ��������֤��������Ϊ������������պ��Ũ����IJ��������������壬���а������ɣ���֤��������Ϊ����������
CaCl2 +2NH3��+2H2O��ʵ���Ҽ���C�ķ�����ʪ��ĺ�ɫʯ����ֽ�������壬����ֽ��������֤��������Ϊ������������պ��Ũ����IJ��������������壬���а������ɣ���֤��������Ϊ����������
���㣺����Ӧ
������������Ŀ����ѧ������ѧ֪ʶ����ϵ������ֻҪ�������������ʼ��ת��������㲻�ѽ����


 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���֣���֪A��B��C��D��E����Ԫ�ض���Ԫ�����ڱ���ǰ20��Ԫ�أ�ԭ��������������E�����������Ų�ʽΪ4s2��A��B��C��D����Ԫ����Ԫ�����ڱ��е����λ�����±���ʾ��
| ���� | A | ||||||
| B | C | D |
����������Ϣ���ش��������⣺
(1)Ԫ��C��Ԫ�����ڱ��е�λ���� ���� �壻D�ĵ����Ų�ʽΪ�� ��
(2)A��D���⻯���У��е�ϸߵ��� ��ԭ����
��A��B�������У��뾶��С���� �������ӷ��ţ���
 (3) A��E��������ӻ�����侧��(�������ھ����о��д����Ե���С�ظ���Ԫ)�ṹ����ͼ��ʾ���������á���ʾ��λ�ڸ�������Ķ�������ģ��������á�������ʾ����λ��С���������ģ����û�����ĵ���ʽ�� ��
(3) A��E��������ӻ�����侧��(�������ھ����о��д����Ե���С�ظ���Ԫ)�ṹ����ͼ��ʾ���������á���ʾ��λ�ڸ�������Ķ�������ģ��������á�������ʾ����λ��С���������ģ����û�����ĵ���ʽ�� ��
��4��A��E������ľ���1/8�����Ϊ2.0��10-23cm3����A��E��ɵ����ӻ�������ܶȣ�����ʽ�����㣨�������һλС������_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ÿ��1�֣���10�֣���֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����硣���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ ��
��2��B���⻯��ķ��ӿռ乹���� ��������ԭ�Ӳ�ȡ �ӻ���
��3��д��������AC2�ĵ���ʽ ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ ����һ��ֻ��B��ɵ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ ��
��4��E�ĺ�������Ų�ʽ�� ��ECl3�γɵ������Ļ�ѧʽΪ ��
��5��D���ʾ���ѻ�Ϊ____________________���á��ʾDԭ�ӣ��ڷ����ڻ����侧��ͼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
��ÿ��1�֣���10�֣���֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����硣���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ ��
��2��B���⻯��ķ��ӿռ乹���� ��������ԭ�Ӳ�ȡ �ӻ���
��3��д��������AC2�ĵ���ʽ ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ ����һ��ֻ��B��ɵ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ ��
��4��E�ĺ�������Ų�ʽ�� ��ECl3�γɵ������Ļ�ѧʽΪ ��
��5��D���ʾ���ѻ�Ϊ____________________���á��ʾDԭ�ӣ��ڷ����ڻ����侧��ͼ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ��һ��һѧ�����п��Ի�ѧ�Ծ� ���ͣ������
����С������10�֣���֪A ��һ����Է�������Ϊ28����̬��������AΪ��Ҫԭ�Ϻϳ�һ�־��й���ζ������E����ϳ�·������ͼ��ʾ����֪B��D�dz������õ�ζ����

��ش��������⣺
��1��д��A�Ľṹ��ʽ ��
��2��B��D�����еĹ��������Ʒֱ��� �� ��
��3������A���Ա�ֱ������ΪD���仯ѧ����ʽ�ɱ�ʾΪ ��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ
�� ��
�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10�����һ�и�һ��ѧ�����п��Ի�ѧ�� ���ͣ������
����С������10�֣���֪A ��һ����Է�������Ϊ28����̬��������AΪ��Ҫԭ�Ϻϳ�һ�־��й���ζ������E����ϳ�·������ͼ��ʾ����֪B��D�dz������õ�ζ����

��ش��������⣺
��1��д��A�Ľṹ��ʽ ��
��2��B��D�����еĹ��������Ʒֱ��� �� ��
��3������A���Ա�ֱ������ΪD���仯ѧ����ʽ�ɱ�ʾΪ ��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ
�� ��
�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com