
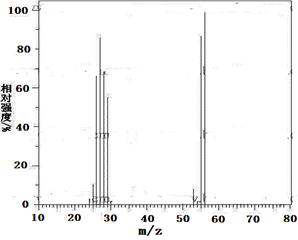
iŅŃÖŖAŹĒĘųĢ¬Ģž£¬ĶźČ«Č¼ÉÕŹ±²śÉśµÄCO2ŗĶH2O µÄĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ1£¬AµÄĻą¶Ō·Ö×ÓÖŹĮæŠ”ÓŚ30£¬ŌŚĻĀĶ¼±ä»ÆÖŠ£¬FĪŖøß·Ö×Ó»ÆŗĻĪļ£¬CÖŠŗ¬ÓŠ-CHO£¬EÓŠĖ®¹ūµÄĻćĪ¶£Ø·“Ó¦Ģõ¼žĪ“Š“³ö£©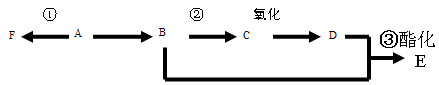
¢Å BÖŠĖłŗ¬¹ŁÄÜĶÅĆū³Ę E ĪļÖŹµÄĆū³Ę
¢Ę ·“Ó¦¢ŁĄąŠĶĪŖ
¢Ē Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£Ø×¢Ć÷·“Ó¦Ģõ¼ž£©
¢Ś ¢Ū
¢¢ Ņ»¶ØĮæµÄŅŅ“¼ŌŚŃõĘų²»×ćµÄĒéæöĻĀČ¼ÉÕ£¬µĆµ½CO”¢CO2ŗĶĖ®µÄ×ÜÖŹĮæĪŖ27.6g,ČōĘäÖŠĖ®µÄÖŹĮæĪŖ10.8g,ŌņCOµÄÖŹĮæĪŖ g.
¢£ÓŠ»śĪļAæÉŅŌĶعż²»Ķ¬»Æѧ·“Ó¦·Ö±šÖʵĆB”¢CŗĶDČżÖÖĪļÖŹ£¬½į¹¹¼ņŹ½ČēĻĀĶ¼ĖłŹ¾”£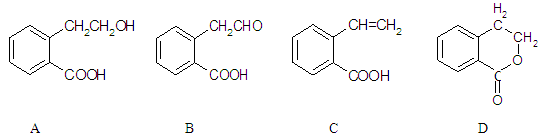
£Ø1£©BÖŠµÄŗ¬Ńõ¹ŁÄÜĶÅĆū³ĘŹĒ ”£
£Ø2£© A”śCµÄ·“Ó¦ĄąŠĶŹĒ £»A”«DÖŠ»„ĪŖĶ¬·ÖŅģ¹¹ĢåµÄŹĒ ”£
£Ø3£©ÓÉAÉś³ÉCµÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø4£©CŌŚŅ»¶ØĢõ¼žĻĀ·¢Éś¼Ó¾Ū·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
 ¶į¹Ś½š¾ķČ«ÄÜĮ·æ¼ĻµĮŠ“š°ø
¶į¹Ś½š¾ķČ«ÄÜĮ·æ¼ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠ·“Ó¦ÖŠ£¬ŹōÓŚ¼Ó³É·“Ó¦µÄŹĒ
| A£®ŅŅĻ©Ź¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ« | B£®½«±½µĪČėäåĖ®ÖŠ£¬Õńµ“ŗóĖ®²ć½Ó½üĪŽÉ« |
| C£®ŅŅĻ©Ź¹äåĖ®ĶŹÉ« | D£®¼×ĶéÓėCl2»ģŗĻ£¬¹āÕÕŗó»ĘĀĢÉ«ĻūŹ§ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø18·Ö£©ßÅßįĪō·Ó (idoxifene)æÉÓĆÓŚÖĪĮĘ¹ĒÖŹŹčĖÉÖ¢£¬ĖüµÄŗĻ³ÉĀ·ĻßČēĶ¼”£
£Ø1£©»ÆŗĻĪļAµÄ·Ö×ÓŹ½ŹĒ £¬1molA×ī¶ąÄÜÓė H2·¢Éś·“Ó¦
£Ø2£©·“Ó¦ĄąŠĶ£ŗB”śC £» D”śE
£Ø3£©DÖŠŗ¬Ńõ¹ŁÄÜĶÅÓŠ£ŗ £ØŠ“Ćū³Ę£©”£
£Ø4£©»ÆŗĻĪļEÄÜ·¢ÉśµÄ·“Ó¦ĄąŠĶŹĒ £ØĢīČėŠņŗÅ£©
| A£®¼Ó³É·“Ó¦ | B£®õ„»Æ·“Ó¦ | C£®Ė®½ā·“Ó¦ | D£®¼Ó¾Ū·“Ó¦ |
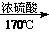 CH2=CH2
CH2=CH2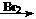 CH2Br-CH2Br
CH2Br-CH2Br²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓŠ»śĪļA”¢B”¢C”¢D”¢E”¢F”¢G”¢HĻą»„×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾”£5.2 g FÄÜÓė100 mL 1 mol/L NaOHČÜŅŗĒ”ŗĆĶźČ«ÖŠŗĶ£¬0.1 mol F»¹ÄÜÓė×ćĮæNaHCO3·“Ó¦£¬ŌŚ±ź×¼×“æöĻĀ·Å³ö4.48 L CO2”£DµÄ·Ö×ÓŹ½ĪŖC3H3O2Na£¬EµÄ·Ö×ÓÖŠŗ¬ÓŠōČ»ł”£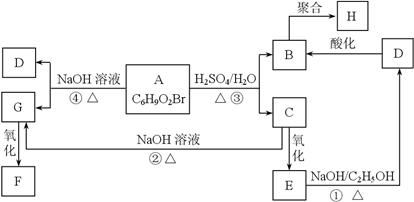
£Ø1£©Š“³öĪļÖŹCÖŠµÄ¹ŁÄÜĶŵÄĆū³Ę£ŗ £»
£Ø2£©Š“³öĪļÖŹF”¢HµÄ½į¹¹¼ņŹ½£»
F ”¢H £»
£Ø3£©Š“³ö·“Ó¦¢Ł”¢¢ÜµÄ»Æѧ·“Ó¦ĄąŠĶ£ŗ¢Ł ”¢¢Ü £»
£Ø4£©Š“³ö±ä»Æ¢Ł”¢¢ŪµÄ»Æѧ·½³ĢŹ½£»
¢Ł
¢Ū
£Ø5£©Š“³öĻą¶Ō·Ö×ÓÖŹĮæ±ČB“ó14£¬ĒŅÓėB¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄĖłÓŠĪļÖŹµÄ½į¹¹Ź½£Ø²»æ¼ĀĒĮ¢ĢåŅģ¹¹£©”£
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø7·Ö£©Ą“×ŌŹÆÓĶµÄÓŠ»ś»Æ¹¤ŌĮĻA£¬Ęä²śĮæŅŃ×÷ĪŖŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½µÄ±źÖ¾£¬AæÉŅŌ·¢ÉśČēĻĀ×Ŗ»Æ£ŗ
ŅŃÖŖ£ŗEŹĒ¾ßÓŠ¹ūĻćĪ¶µÄÓŠ»śĪļ£¬Ęä·Ö×ÓŹ½ĪŖC4H8O2£¬FŹĒŅ»ÖÖøß·Ö×Ó»ÆŗĻĪļ”£
£Ø1£©AµÄ·Ö×ÓŹ½ŹĒ””””””””””””””””£¬CµÄĆū³ĘŹĒ”””””””””””””””£
£Ø2£©D·Ö×ÓÖŠµÄ¹ŁÄÜĶÅĆū³ĘŹĒ””””””””£¬Ö¤Ć÷øĆ¹ŁÄÜĶžßÓŠĖįŠŌµÄ·½·ØŹĒ ”””””””””””£
£Ø3£©·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ŹĒ £»·“Ó¦¢ÜµÄĄąŠĶŹĒ ·“Ó¦”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
[»Æѧ”ŖÓŠ»ś»Æѧ»ł“”]£Ø13·Ö£©
Ä³ŃŠ¾æŠ”×éŅŌ¼×±½ĪŖÖ÷ŅŖŌĮĻ£¬²ÉÓĆŅŌĻĀĀ·ĻßŗĻ³ÉŅ½Ņ©ÖŠ¼äĢåFŗĶY”£
ŅŃÖŖ£ŗ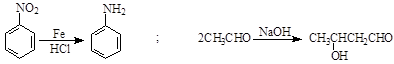 ”£
ӣ
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öYÖŠŗ¬Ńõ¹ŁÄÜĶŵÄĆū³Ę ”£
£Ø2£©ĻĀĮŠÓŠ¹ŲFµÄĖµ·ØÕżČ·µÄŹĒ ”£
| A£®·Ö×ÓŹ½ŹĒC7H7NO2Br | B£®¼ČÄÜÓėŃĪĖį·“Ó¦ÓÖÄÜÓėNaOHČÜŅŗ·“Ó¦ |
| C£®ÄÜ·¢Éśõ„»Æ·“Ó¦ | D£®1 mol F×ī¶ąæÉŅŌĻūŗÄ2 mol NaOH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŅĻ©ŹĒŹÆÓĶ»Æ¹¤×īÖŲŅŖµÄ»ł“”ŌĮĻ£¬Ēėøł¾ŻŅŌĻĀæņĶ¼»Ų“š£ŗ
41.ŅŌŹÆÓĶĪŖŌĮĻµÄĻµĮŠ»Æ¹¤Éś²ś¹ż³ĢÖŠ£¬µĆµ½“óĮæŅŅĻ©µÄÖ÷ŅŖ·½·ØŹĒ_______£ØŃ”ĢīŠņŗÅ£©”£
a. Ė®½ā b. ·ÖĮó c. ĮŃ½ā d. ĮŃ»Æ
42.ÓŠ»śĪļAĖ׳Ę______________£¬ŗ¬ÓŠµÄ¹ŁÄÜĶÅĆū³ĘŹĒ_________________.
43.BµÄ·Ö×ÓŹ½ĪŖC2H4O2£¬Óė“æ¼ī·“Ó¦ÄÜÉś³É¶žŃõ»ÆĢ¼ĘųĢ壬Š“³ö·“Ó¦A£«B”śCµÄ»Æѧ
·½³ĢŹ½___________________________________________________________ £ØÓŠ»śĪļÓĆ½į¹¹¼ņŹ½±ķŹ¾£©£¬øĆ·“Ó¦ĄąŠĶĪŖ______________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø18·Ö£©ĻÖÓŠ2.8gÓŠ»śĪļA£¬ĶźČ«Č¼ÉÕÉś³É0.15molCO2ŗĶ1.8gH2O£¬AµÄÖŹĘ×Ķ¼ČēÓŅĶ¼ĖłŹ¾£ŗŅŃÖŖ£ŗ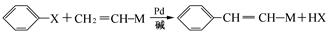 £ØXĪŖĀ±Ō×Ó£¬MĪŖĢž»ł»ņŗ¬õ„»łµÄČ”“ś»łµČ)£¬ÓÉÓŠ»śĪļAŗĻ³ÉG£ØĻć¶¹ĖŲ£©µÄ²½ÖčČēĻĀ£ŗ
£ØXĪŖĀ±Ō×Ó£¬MĪŖĢž»ł»ņŗ¬õ„»łµÄČ”“ś»łµČ)£¬ÓÉÓŠ»śĪļAŗĻ³ÉG£ØĻć¶¹ĖŲ£©µÄ²½ÖčČēĻĀ£ŗ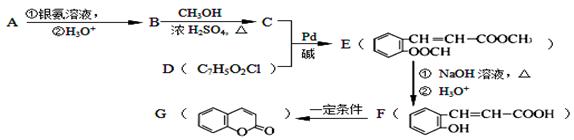
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AµÄ·Ö×ÓŹ½ĪŖ ”£
£Ø2£©Š“³öCÖŠŗ¬Ńõ¹ŁÄÜĶÅĆū³Ę:”” ””£»F”śG µÄ·“Ó¦ĄąŠĶŹĒ ”£
£Ø3£©Š“³öAŗĶŅų°±ČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½”” ”””£
£Ø4£©DµÄ½į¹¹¼ņŹ½ĪŖ”” ”””£
£Ø5£©¶žĒāĻć¶¹ĖŲ£Ø  £©³£ÓĆ×÷Ļć¶¹ĖŲµÄĢꓜʷ£¬¼ų±š¶žĒāĻć¶¹ĖŲŗĶĖüµÄŅ»ÖÖĶ¬·ÖŅģ¹¹Ģå£Ø
£©³£ÓĆ×÷Ļć¶¹ĖŲµÄĢꓜʷ£¬¼ų±š¶žĒāĻć¶¹ĖŲŗĶĖüµÄŅ»ÖÖĶ¬·ÖŅģ¹¹Ģå£Ø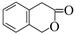 £©ŠčŅŖÓƵ½µÄŹŌ¼ĮÓŠ£ŗNaOHČÜŅŗ”¢”” ”””£
£©ŠčŅŖÓƵ½µÄŹŌ¼ĮÓŠ£ŗNaOHČÜŅŗ”¢”” ”””£
£Ø6£©FÓŠ¶ąÖÖĶ¬·ÖŅģ¹¹Ģ壬Š“³öĶ¬Ź±Āś×ćĻĀĮŠĢõ¼žµÄĮ½ÖÖĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ ”””£
¢ń. ·Ö×ÓÖŠ³ż±½»·Ķā£¬ĪŽĘäĖü»·×“½į¹¹£» ¢ņ.±½»·ÉĻÓŠĮ½øö“¦ÓŚ¶ŌĪ»µÄČ”“ś»ł£»
¢ó. ÄÜ·¢ÉśĖ®½ā·“Ó¦£¬²»ÄÜÓėNa·“Ó¦£» ¢ō.ÄÜÓėŠĀÖĘCu(OH)2°“ĪļÖŹµÄĮæ±Č1:2·¢ÉśŃõ»Æ·“Ó¦
£Ø7£©ÓÖÖŖ£ŗ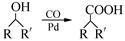 (R£¬R”äĪŖĢž»ł)£¬ŹŌŠ“³öŅŌ±½ŗĶ±ūĻ©£Ø
(R£¬R”äĪŖĢž»ł)£¬ŹŌŠ“³öŅŌ±½ŗĶ±ūĻ©£Ø £½CH”ŖCH3£©ĪŖŌĮĻ£¬ŗĻ³É
£½CH”ŖCH3£©ĪŖŌĮĻ£¬ŗĻ³É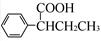 µÄĀ·ĻßĮ÷³ĢĶ¼ČēĻĀ£ŗ
µÄĀ·ĻßĮ÷³ĢĶ¼ČēĻĀ£ŗ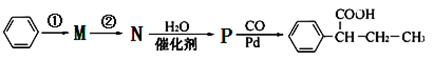
²½Öč¢ŁµÄ·“Ó¦Ģõ¼žŗĶŹŌ¼Į____________£¬²½Öč¢ŚµÄ·“Ó¦ĄąŠĶ____________£¬PµÄ½į¹¹¼ņŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Õ³ŗĻ¼ĮMµÄŗĻ³ÉĀ·ĻßČēĻĀĶ¼ĖłŹ¾£ŗ
Ķź³ÉĻĀĮŠĢīæÕ£ŗ
£Ø1£©Š“³öAŗĶBµÄ½į¹¹¼ņŹ½”£A””””””””£¬B”””””””””£
£Ø2£©Š“³ö·“Ó¦Ģõ¼ž£ŗ·“Ó¦¢Ś””””””””£»·“Ó¦¢Ż”””””””””£
£Ø3£©·“Ó¦¢ŪŗĶ¢ŻµÄÄæµÄŹĒ___________________________________________”£
£Ø4£©CµÄ¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄĶ¬·ÖŅģ¹¹Ģå¹²ÓŠ””””””””ÖÖ”£
£Ø5£©Š“³öDŌŚ¼īŠŌĢõ¼žĻĀĖ®½āµÄ·“Ó¦·½³ĢŹ½£ŗ___________________________
__________________________________________________________________
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com