
����Ŀ������(�ֳ��£�N2H4����ɫҺ��)��һ��Ӧ�ù㷺�Ļ���ԭ�ϣ����������ȼ��������ƽ�����װ�л�ԭ����(N2H4)��ǿ��������������(H2O2)�������ǻ��ʱ������������������ˮ���������ų������ȡ���֪0.5 molҺ̬���������������ⷴӦ�����ɵ�����ˮ�������ų�320.8 kJ��������
��1���µĵ���ʽΪ___________________����������ĵ���ʽΪ__________________��
��2��д����Ӧ���Ȼ�ѧ����ʽ��________________________________________________��
��3����25 �桢101 kPaʱ����֪18 gˮ�������Һ̬ˮ�ų�44 kJ����������������������±���
O===O | H��H | H��O(g) | |
1 mol��ѧ������ʱ ��Ҫ���յ�����/kJ | 496 | 436 | 463 |
д����ʾH2ȼ���ȵ��Ȼ�ѧ����ʽ___________________________________________����32 gҺ̬��������Һ̬�������ⷴӦ���ɵ�����Һ̬ˮʱ���ų���������________kJ��
��4��������H2O2����Ϊ����ƽ�������Ҫԭ��Ϊ_________________________________��
��5������Ϊ��Ԫ�����ˮ�еĵ��뷽��ʽ�백���ƣ�������һ�����뷴Ӧ��ƽ�ⳣ��ֵΪ____________(��֪��N2H4+H+![]() N2H5+��K=8.7��107��KW=1.0��10-14)�������������γɵ���ʽ�εĻ�ѧʽΪ_______________��
N2H5+��K=8.7��107��KW=1.0��10-14)�������������γɵ���ʽ�εĻ�ѧʽΪ_______________��
���𰸡�![]()
![]() N2H4(l)��2H2O2(l)===N2(g)��4H2O(g) ��H����641.6 kJ��mol��1 H2 (g) +
N2H4(l)��2H2O2(l)===N2(g)��4H2O(g) ��H����641.6 kJ��mol��1 H2 (g) + ![]() O2 (g) = H2O (l) ��H= -286 kJmol-1 817.6 ��Ӧ�����������������壨������N2��H2O���Ի�������Ⱦ�� 8.7��10-7 N2H6(HSO4)2
O2 (g) = H2O (l) ��H= -286 kJmol-1 817.6 ��Ӧ�����������������壨������N2��H2O���Ի�������Ⱦ�� 8.7��10-7 N2H6(HSO4)2
��������
(1)�·���ʽΪN2H4��ÿ����ԭ���γ�������ѧ���������������ʽH2O2��ÿ����ԭ���γ��������ۼ����ݴ�д�����ʵĵ���ʽ��
(2)�����Ȼ�ѧ����ʽ��д����д������ע���ʾۼ�״̬�ͷ�Ӧ�ʱ���
(3) ��Ӧ����ܼ���-��������ܼ���=��Ӧ�����ݴ˼��㷴Ӧ����д���Ȼ�ѧ����ʽ�������Ȼ�ѧ����ʽ��˹���ɼ���õ��Ȼ�ѧ����ʽ���õ���Ӧ���ʱ���
(4)���ṩ�����Ͷ����Լ���������Ⱦ�Ƕȷ�����
(5) ����Ϊ��Ԫ��������ˮ�еĵ��뷽ʽ�백������������һ�����뷽��ʽΪN2H4+ H2O ![]() N2H5++OH-��ƽ�ⳣ��Kb=
N2H5++OH-��ƽ�ⳣ��Kb=![]() =
=![]() ��
��![]() =K��Kw��
=K��Kw��
��Ϊ�Ƕ�Ԫ������������������γɵ���ʽ��ΪN2H6(HSO4)2��
(1)���ǵ�ԭ�Ӻ͵�ԭ���γ�һ�����ۼ���ʣ��ۼ�����ԭ���γɹ��ۼ�������ʽΪ![]() ��˫��ˮΪ���ۻ������������д��������������һ��O-O����˫��ˮ�ĵ���ʽΪ��
��˫��ˮΪ���ۻ������������д��������������һ��O-O����˫��ˮ�ĵ���ʽΪ��![]() ��
��
��ˣ�������ȷ������![]() ��
��![]() ��
��
(2) 0.5 molҺ̬���������������ⷴӦ�����ɵ�����ˮ�������ų�320.8 kJ��������1mol��ȼ�շ���Ϊ641.6 kJ����ȼ�յ��Ȼ�ѧ����ʽΪ��N2H4(l)��2H2O2(l)===N2(g)��4H2O(g) ��H����641.6 kJ��mol��1��
��ˣ�������ȷ������N2H4(l)��2H2O2(l)===N2(g)��4H2O(g) ��H����641.6 kJ��mol��1��
(3) ���ݷ�Ӧ����ܼ���-��������ܼ���=��Ӧ�ȣ���1mol����ȼ������1molˮ�����ķ�Ӧ��Ϊ436kJ/mol+496kJ/mol��![]() -463kJ/mol��2=-242kJ/mol��18gˮ�������Һ̬ˮ�ų�44kJ����������1mol����ȼ������1molҺ̬ˮʱ�ų�����Ϊ242kJ+44kJ=286kJ��
-463kJ/mol��2=-242kJ/mol��18gˮ�������Һ̬ˮ�ų�44kJ����������1mol����ȼ������1molҺ̬ˮʱ�ų�����Ϊ242kJ+44kJ=286kJ��
�ʱ�ʾH2ȼ���ȵ��Ȼ�ѧ����ʽΪH2 (g) + ![]() O2 (g) = H2O (l) ��span>H= -286 kJmol-1��
O2 (g) = H2O (l) ��span>H= -286 kJmol-1��
��֪��N2H4(l)��2H2O2(l)===N2(g)��4H2O(g) ��H����641.6 kJ��mol��1��
��H2O(l)�TH2O(g) ��H��+44kJ��mol-1�����ݸ�˹��������-�ڡ�4�õ���N2H4(l)��2H2O2(l)===N2(g)��4H2O(l) ��H����817.6 kJ��mol��1��
��32gҺ̬��������Һ̬�������ⷴӦ���ɵ�����Һ̬ˮ����Ϊ817.6 kJ��
��ˣ�������ȷ������H2 (g) + ![]() O2 (g) = H2O (l) ��H= -286 kJmol-1 ��817.6��
O2 (g) = H2O (l) ��H= -286 kJmol-1 ��817.6��
��4��N2H4����ǿ��ԭ�Ժ�H2O2����������ԭ��Ӧ�ų��������Ҳ����������壬��˿���Ϊ����ƽ�����
��ˣ�������ȷ��������Ӧ�����������������壨������N2��H2O���Ի�������Ⱦ����
��5������Ϊ��Ԫ��������ˮ�еĵ��뷽ʽ�백������������һ�����뷽��ʽΪN2H4+ H2O ![]() N2H5++OH-��ƽ�ⳣ��Kb=
N2H5++OH-��ƽ�ⳣ��Kb=![]() =
=![]() ��
��![]() =K��Kw=8.7��107��1.0��10-14=8.7��10-7��
=K��Kw=8.7��107��1.0��10-14=8.7��10-7��
��Ϊ�Ƕ�Ԫ������������������γɵ���ʽ��ΪN2H6(HSO4)2��
��ˣ�������ȷ������8.7��10-7 ��N2H6(HSO4)2��


 ����������������ϵ�д�
����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й����ʵ�ת����ϵ����ͼ��ʾ(������������������ȥ)������D��F��GΪ���������壬DΪ���ʣ�F��GΪ�����GΪ�غ�ɫ��BΪ����ɫ���壬X�������Һ�壬Y�dz����Ľ������ʣ�I��ҺΪ��ɫ��

��ش��������⣺
��1��B�Ļ�ѧʽΪ____��
��2��X�ĵ���ʽΪ____��
��3��д����Ӧ�ٵĻ�ѧ����ʽ��__________________________��
��4��д����Ӧ�ڵ����ӷ���ʽ(��ӦʱHΪϡ��Һ)��__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��G��ǰ�����ڳ�ϡ������֮��ԭ�������������������Ԫ�ء�A������Ԫ�ؼȲ�ͬ�����ֲ�ͬ�壻B��C�ļ۵��Ӳ���δ�ɶԵ���������2��E�����s��p�ܼ��ĵ���������ȣ�F��Eͬ�����ҵ�һ�����ܱ�EС��G�ģ�1�����ӣ�G+���ĸ������ȫ�������ش��������⣺
��1��д��Ԫ�����ƣ�B_______��G_______��
��2��д��F�ļ۵����Ų�ͼ��_______��
��3��д��������BC�Ľṹʽ��__________________��
��4����A��C��F��Ԫ���γɵ�����[F(CA)4]�� ��F��ˮ��Һ�е�һ�ִ�����ʽ������F���ӻ�������________________��
��5���ڲⶨA��D�γɵĻ��������Է�������ʱ��ʵ��ⶨֵһ���������ֵ����Ҫԭ����______________________��
��6��E��һ�־���ṹ��ͼ�ף�����һ�������к���_______��E��G��D�γɵĻ�����ľ����ṹ��ͼ�ң��������ܶ�Ϊag/cm3����G��D����ľ���Ϊ____pm
�������ӵ�������NA��ʾ���г��������ʽ�����û���������ΪG����ΪD����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����25mL 0.1mol/L NaOH ��Һ����μ���0.2mol/L CH3COOH��Һ��������ͼ��ʾ�������й�����Ũ�ȹ�ϵ�ıȽϣ���ȷ����

A. A��B ֮������һ�㣬��Һ��һ������c(Na��)>c(CH3COO��)>c(OH��)>c(H��)
B. B�㣬a��12.5������c(Na��)=c(CH3COO��)>c(OH��)>c(H��)
C. C�㣺c(Na��)>c(CH3COO��)> c(H��) > c(OH��)
D. D�㣺c(CH3COO-)��c(CH3COOH)��2c(Na+ )
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ���Ҳ����ķ�Һ�к���Fe3+��Cu2+��Ba2+���ֽ������ӣ��о�С����������з����Է�Һ���д������Ի��ս���������������

��ش�
(1)����٢ڢ��õ�����Ҫ����������___________________��
(2)����۵�ʵ��������_________________��
(3) ���һ��ʵ�鷽������֤�����������������Һ��������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͪ�����һ�����õĿ�����ʹҩ������ͨ�����·����ϳɣ�

��1��ͪ����к��������ŵ�����Ϊ______________��______________��
��2����B��C�ķ�Ӧ������____________________��
��3��д��D��E��Ӧ�Ļ�ѧ����ʽ______________________________________________��
��4��д��ͬʱ��������������A��һ��ͬ���칹��Ľṹ��ʽ_____________________��
��.�ܷ���������Ӧ
��.ˮ�����֮һ��FeCl3��Һ��ɫ
��.�����к���4�ֲ�ͬ��ѧ��������ԭ��
��5����д���Լױ����Ҵ�Ϊԭ���Ʊ�������![]() �ĺϳ�·������ͼ�����Լ�����ѡ��________________��
�ĺϳ�·������ͼ�����Լ�����ѡ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���¶��µĶ����ܱ������У��������������ӦA(s)��2B(g)![]() C(g)+D(g) �Ѵﵽ��Ӧ�ȵ���
C(g)+D(g) �Ѵﵽ��Ӧ�ȵ���
A. ���������ܶȱ��ֲ���B. ��1mol C���ɵ�ͬʱ��1mol D����
C. �������������ʵ������ֲ���D. ��1 mol A���ɵ�ͬʱ��1mol C����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ѱ���Ϊ�����������㷺���ں��캽�յ���������VB2��-������صķŵ練ӦΪ4VB2+11O2===4B2O3+2V2O5���Ըõ��Ϊ��Դ�Ʊ��ѵ�װ����ͼ��ʾ��
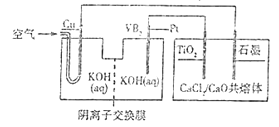
����˵����ȷ���ǣ� ��
A. �������У�OH-�������ӽ���Ĥ�Ҳ������Ǩ��
B. Pt����ӦʽΪ2VB2+22OH--22e-===V2O5+2B2O3+11H2O
C. �������У�ͭ�������������Һ��pH��С
D. ��ʯī��ֻ�ռ���4.48LCl2���壬���������Ʊ�4.8gTi
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�Һϳɳ���һ���»�����(��ͼ��ʾ)������W��X��Y��ZΪͬһ������Ԫ�أ�Z����������������X�����������һ�롣������������ȷ����
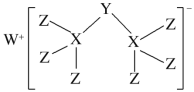
A.W����������������ӻ�����
B.���»�������X����8�����ȶ��ṹ
C.Ԫ�طǽ����Ե�˳��ΪX��Y��Z
D.Z������������ˮ������ǿ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com