
���н���ʵ������ķ�Ӧ����ʽ��ȷ����(����)
A���п��Ľ���Na��¶�ڿ����У����������䰵2Na��O2===Na2O2
B����AgCl����Һ�еμ�Na2S��Һ����ɫ������ɺ�ɫ2AgCl��S2��=Ag2S����2Cl��
C��Na2O2�ڳ�ʪ�Ŀ����з���һ��ʱ�䣬��ɰ�ɫ����2Na2O2��2CO2=2Na2CO3��O2
D����NaHCO3��Һ�м�������ij���ʯ��ˮ�����ְ�ɫ����2HCO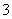 ��Ca2����2OH��=CaCO3����CO
��Ca2����2OH��=CaCO3����CO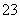 ��2H2O
��2H2O
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ɫ��Һ������K2CO3��MgCl2��NaHCO3��BaCl2��Һ�е�һ�ֻ�����ɡ�����Һ�м����ռ���Һ���ְ�ɫ����������ϡ����Ҳ���ְ�ɫ�������ų����塣�ݴ˷����������ж�����ȷ���ǣ�������
�ٿ϶���BaCl2�� �ڿ϶���MgCl2�� �ۿ϶���NaHCO3��
�ܿ϶���Na2CO3��NaHCO3���ݿ϶�û��MgCl2
A���٢ڢ� B���ڢ� C���٢� D���٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ϊm1��NaHCO3�����þƾ��Ƽ���һ��ʱ����ʣ����������Ϊm2 ��
��1��������Ӧ��NaHCO3����Ϊ���٣�
��2����ʣ����������Ϊ����ʱ�����Զ϶�NaHCO3�ѷֽ���ȫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijpH��1�Ĺ�ҵ��Һ��ֻ���ܺ������������е������֣�H����Mg2����Ba2����Cl����CO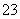 ��SO
��SO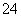 ����ȡ����100 mL��Һ��������ʵ�飺
����ȡ����100 mL��Һ��������ʵ�飺
��һ�ݼ�������AgNO3��Һ���ø������3. 50 g��
50 g��
�ڶ��ݼ�������BaCl2��Һ�ø������2.33 g������������ϴ�ӡ���������������䡣
��������ʵ�飬�����Ʋ���ȷ���� (����)
��һ������Mg2�����ڿ��ܴ���CO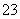 ����һ������Cl�����ܿ��ܴ���Ba2�����ݿ��ܴ���Mg2��
����һ������Cl�����ܿ��ܴ���Ba2�����ݿ��ܴ���Mg2��
A���٢� B���ڢ�
C���ۢ� D���ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ɫ��Һ�к���K����Cl����OH����SO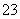 ��SO
��SO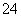 ��Ϊ������Һ��������ijЩ�����ӣ����õ��Լ��У����ᡢ���ᡢ��������Һ�����ᱵ��Һ����ˮ�ͷ�̪��Һ����������OH����ʵ�鷽��ʡ�ԣ��������������ӵĹ�������ͼ��ʾ��
��Ϊ������Һ��������ijЩ�����ӣ����õ��Լ��У����ᡢ���ᡢ��������Һ�����ᱵ��Һ����ˮ�ͷ�̪��Һ����������OH����ʵ�鷽��ʡ�ԣ��������������ӵĹ�������ͼ��ʾ��
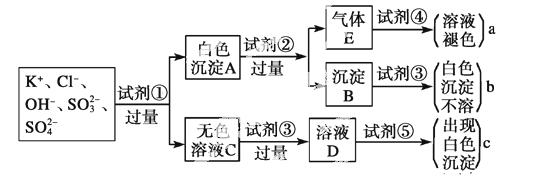
(1)ͼ���Լ��١������ʵĻ�ѧʽ�ֱ���
��________����________����________����__________��
��__________��
(2)ͼ������a��b��c��������������ӷֱ���
a________��b___ _____��c________��
_____��c________��
(3)��ɫ����A���Լ��ڷ�Ӧ�����ӷ���ʽ��_________________________________
________________________________________________________________________��
(4)��ɫ��ҺC���Լ��۵���ҪĿ����___________________________ __________��
__________��
(5)��ɫ����A�����Լ��۶������Լ��ڣ���ʵ���Ӱ����____________________��
(6)����Eͨ���Լ��ܷ�����Ӧ�����ӷ���ʽ��_______________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���н�����ʵ�ķ���ʽ����ȷ���ǣ� ��
A����0.1mol/L��ˮ��pHΪ11��NH3·H2O NH4++OH-
NH4++OH-
B����Na�����ˮ�У��������壺2Na+2H2O=2NaOH +H2��
+H2��
C����CuCl2��Һ������ʵ�飬���ݷ��⣺CuCl2 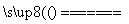 Cu2++2Cl-
Cu2++2Cl-
D��AlƬ����NaOH��Һ�У��������壺2Al+2OH-+2H2O=2AlO2-+3H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȷ��ʾ���з�Ӧ�����ӷ�Ӧ����ʽΪ
A��NH4HCO3���ڹ�����ŨKOH��Һ�У�
NH4++ HCO3-+2OH-= CO32-+ NH3��+2 H2O
B����������Һ�еμ�Ba(OH)2��Һ��ǡ��ʹSO42-������ȫ��
2Al3++3SO42-+3Ba2++6OH -=2 Al(OH)3��+3BaSO4��
C����FeBr2��Һ��ͨ������������2Fe2++4Br-+3Cl2=2 Fe3++2 Br2+6 Cl-
D�������ȥˮ����2H++CaCO3=Ca2++ CO2��+ H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�����ӷ���ʽ��д��ȷ���� (����)��
A����Al��Ͷ��NaOH��Һ�У�Al��OH����H2O=AlO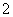 ��H2��
��H2��
B��ͭ����ϡ�����У�Cu��4H����2NO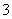 =Cu2����2NO2����2H2O
=Cu2����2NO2����2H2O
C��̼�������Һ�м������������������Һ��HCO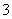 ��OH��=CO
��OH��=CO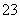 ��H2O
��H2O
D����̼������Һ����μ�����֮����������ʵ���Ũ�ȵ�ϡ���
CO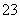 ��CH3COOH=CH3COO����HCO
��CH3COOH=CH3COO����HCO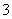
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�����������˵����ȷ����(����)
A����״���£�22.4 L C8H18�к��еĹ��ۼ�����Ϊ25NA
B��56 g Fe��71 g Cl2��ַ�Ӧ��ת����2NA��e��
C��22.4 L NO��22.4 L O2��ֻ�Ϻ�������к���3NA��Oԭ��
D��1 mol/L��MgCl2��Һ�к�2NA��Cl��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com