
�����£���20mL 0.2mol/L H2A��Һ�еμ�0.2 mol/L NaOH��Һ���й��������ʵ����仯����ͼ������I����H2A��II����HA-��III����A2-���� ����ͼʾ�жϣ�����˵����ȷ����
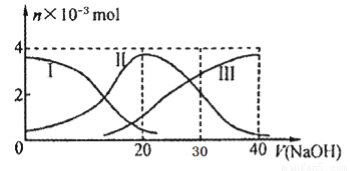
A��H2A��ˮ�еĵ��뷽��ʽ���� H2A? === H+? +? HA��? ��? HA-??????  H+? +? A2��
H+? +? A2��
B����V(NaOH)=20mLʱ����Һ�и�����Ũ�ȵĴ�С˳��Ϊ��c(Na+)��c(HA-)��c(H+)��c(A2-)��c(OH-)
C���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ��
D�� ��V(NaOH)=30mLʱ����Һ�д������¹�ϵ��2c(H+��+ c(HA-��+ 2c(H2A���� c(A2-��+ 2 c(OH-)
B
��������
���������A������ͼ���֪����Һ��H2A��Ũ�����������Ƶļ�����С����˵��H2A�Ƕ�Ԫ���ᣬ����Һ�еĵ��뷽��ʽΪH2A H+ + HA����HA-
H+ + HA����HA-  H+ + A2����A����ȷ��B������ͼ��֪����V��NaOH����20 mLʱ��������ӦΪNaOH+H2A�TNaHA+H2O��������ҪΪNaHA��HA-����̶ȴ���ˮ��̶ȣ���Һ�����ԣ�ˮ��HA-������������ӣ�ֻ��HA-�����A2-����������Ũ�ȴ�С˳����c��Na+����c��HA-����c��H+����c��A2-����c��OH-������B��ȷ��C���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�������ӦΪNaOH+H2A�TNaHA+H2O��������ҪΪNaHA��HA-����̶ȴ���ˮ��̶ȣ���Һ�����ԣ�����Һ��ˮ�ĵ���̶ȱȴ�ˮС��C����ȷ��D����V(NaOH)=30mLʱ�����߷�Ӧ����NaHA��Na2A���Ҷ��ߵ����ʵ���Ũ����ȣ�����ݵ���غ�c��OH-��+2c��A2-��+c��HA-����c��H+��+c��Na+���������غ�c2��Na+��=3c��HA-��+3c��H2A��+3c��A2-����֪����Һ�д������¹�ϵ��2c(H+��+ c(HA-��+ 3c(H2A���� c(A2-��+ 2 c(OH-)��D����ȷ����ѡB��
H+ + A2����A����ȷ��B������ͼ��֪����V��NaOH����20 mLʱ��������ӦΪNaOH+H2A�TNaHA+H2O��������ҪΪNaHA��HA-����̶ȴ���ˮ��̶ȣ���Һ�����ԣ�ˮ��HA-������������ӣ�ֻ��HA-�����A2-����������Ũ�ȴ�С˳����c��Na+����c��HA-����c��H+����c��A2-����c��OH-������B��ȷ��C���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�������ӦΪNaOH+H2A�TNaHA+H2O��������ҪΪNaHA��HA-����̶ȴ���ˮ��̶ȣ���Һ�����ԣ�����Һ��ˮ�ĵ���̶ȱȴ�ˮС��C����ȷ��D����V(NaOH)=30mLʱ�����߷�Ӧ����NaHA��Na2A���Ҷ��ߵ����ʵ���Ũ����ȣ�����ݵ���غ�c��OH-��+2c��A2-��+c��HA-����c��H+��+c��Na+���������غ�c2��Na+��=3c��HA-��+3c��H2A��+3c��A2-����֪����Һ�д������¹�ϵ��2c(H+��+ c(HA-��+ 3c(H2A���� c(A2-��+ 2 c(OH-)��D����ȷ����ѡB��
���㣺����ˮ�ĵ��롢��Һ������Ũ�ȴ�С�Ƚϵ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
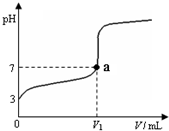 ��2011?�����ж�ģ�������£���20mL x mol?L-1 CH3COOH��Һ����μ�������ʵ���Ũ�ȵ�NaOH��Һ�����Һ��pH��NaOH��Һ�������V���ı仯��ϵ��ͼ��ʾ�������¶ȱ仯��������˵������ȷ���ǣ�������
��2011?�����ж�ģ�������£���20mL x mol?L-1 CH3COOH��Һ����μ�������ʵ���Ũ�ȵ�NaOH��Һ�����Һ��pH��NaOH��Һ�������V���ı仯��ϵ��ͼ��ʾ�������¶ȱ仯��������˵������ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
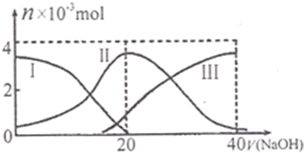
 ��֪������CH3COOH�ĵ���ƽ�ⳣ��ΪKa�������£���20mL 0.1mol?L-1 CH3COOH��Һ����μ���0.1mol?L-1NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵������ȷ���ǣ�������
��֪������CH3COOH�ĵ���ƽ�ⳣ��ΪKa�������£���20mL 0.1mol?L-1 CH3COOH��Һ����μ���0.1mol?L-1NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵������ȷ���ǣ�������| A��a���ʾ����Һ����ˮ�������H+Ũ��Ϊ1.0��10-3mol?L-1 | ||
| B��b���ʾ����Һc��CH3COO-����c��Na+�� | ||
| C��c���ʾCH3COOH��NaOHǡ�÷�Ӧ��ȫ | ||
D��b��d���ʾ����Һ��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
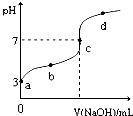
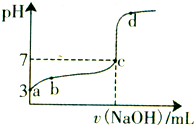 �����£���20mL 0.1moL/L CH3COOH��Һ����μ���0.1mol/L NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵������ȷ���ǣ�������
�����£���20mL 0.1moL/L CH3COOH��Һ����μ���0.1mol/L NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵������ȷ���ǣ�������| A��a���ʾ����Һ��c��OH-��=10-11mol/L | ||
B��a��b���ʾ����Һ��
| ||
| C��c���ʾCH3COOH��NaOHǡ����ȫ��Ӧc��CH3COOH��?c��OH-�� | ||
| D��d���ʾ����Һ��c��Na+����c��CH3COO-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
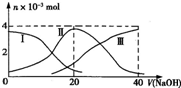 �����£���20mL 0.2mol?L-1 H2SO3����Һ�еμ�0.2mol?L-1 NaOH��Һ���й��������ʵ����仯������ͼ��ʾ��������I����H2SO3�������HS
�����£���20mL 0.2mol?L-1 H2SO3����Һ�еμ�0.2mol?L-1 NaOH��Һ���й��������ʵ����仯������ͼ��ʾ��������I����H2SO3�������HS| O | - 3 |
| O | 2- 3 |
| A����V��NaOH��=0ʱ����ˮ�������c��H+��=1.0��10-12?? | ||||
B����V��NaOH��=20 mLʱ��c��Na+����c��HS
| ||||
C����V��NaOH��=40 mLʱ2c��Na+��=c��S
| ||||
| D����V��NaOH��=40 mL�����μ�NaOH��Һ����Һ���¶Ȼ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com