
”¾ĢāÄæ”æij¹¤³§¶Ō¹¤ŅµÉś²śīŃ°×·Ū²śÉśµÄ·ĻŅŗ½ųŠŠ×ŪŗĻĄūÓĆ£¬·ĻŅŗÖŠŗ¬ÓŠ“óĮæFeSO4”¢H2SO4ŗĶÉŁĮæFe2£ØSO4£©3”¢TiOSO4£¬æÉÓĆÓŚÉś²śŃÕĮĻĢśŗģŗĶ²¹ŃŖ¼ĮČéĖįŃĒĢś£®ĘäÉś²ś¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
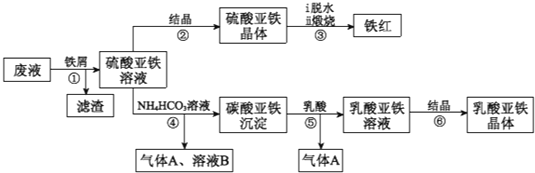
ŅŃÖŖ£ŗ¢ŁTiOSO4æÉČÜÓŚĖ®£¬ŌŚĖ®ÖŠæÉŅŌµēĄėĪŖTiO2+ŗĶSO42-£»
¢ŚTiOSO4Ė®½āµÄ·“Ó¦ĪŖ£ŗTiOSO4+£Øx+1£©H2O”śTiO2xH2O”ż+H2SO4£®
Ēė»Ų“š£ŗ
£Ø1£©²½Öč¢ŁĖłµĆĀĖŌüµÄÖ÷ŅŖ³É·ÖĪŖ_____________£¬
£Ø2£©²½Öč¢ŪĮņĖįŃĒĢśŌŚæÕĘųÖŠģŃÉÕÉś³ÉĢśŗģŗĶČżŃõ»ÆĮņ£¬øĆ·“Ó¦ÖŠŃõ»Æ¼ĮŗĶ»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ_____________£¬
£Ø3£©²½Öč¢ÜŠčæŲÖĘ·“Ó¦ĪĀ¶ČµĶÓŚ35”ę£¬ĘäÄæµÄŹĒ_____________£¬
£Ø4£©²½Öč¢ÜµÄĄė×Ó·½³ĢŹ½ŹĒ_____________£¬
£Ø5£©ŅŃÖŖ£ŗFeCO3£ØS£©![]() Fe2+£Øaq£©+CO32-£Øaq£©£¬ŹŌÓĆĘ½ŗāŅʶÆŌĄķ½āŹĶ²½Öč¢ŻÉś³ÉČéĖįŃĒĢśµÄŌŅņ_____________£¬
Fe2+£Øaq£©+CO32-£Øaq£©£¬ŹŌÓĆĘ½ŗāŅʶÆŌĄķ½āŹĶ²½Öč¢ŻÉś³ÉČéĖįŃĒĢśµÄŌŅņ_____________£¬
£Ø6£©ČÜŅŗB³£±»ÓĆÓŚµē½āÉś²ś£ØNH4£©2S2O8£Ø¹ż¶žĮņĖįļ§£©”£µē½āŹ±¾łÓƶčŠŌµē¼«£¬Ńō¼«·¢ÉśµÄµē¼«·“Ó¦æɱķŹ¾ĪŖ_____________£¬
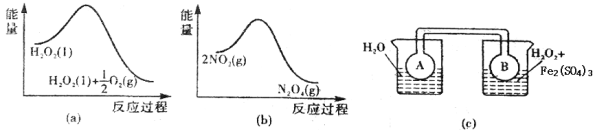
£Ø7£©Fe3+¶ŌH2O2µÄ·Ö½ā¾ßÓŠ“ß»Æ×÷ÓĆ£®ĄūÓĆĶ¼2£Øa£©ŗĶ£Øb£©ÖŠµÄŠÅĻ¢£¬°“Ķ¼2£Øc£©×°ÖĆ£ØĮ¬ĶصÄA”¢BĘæÖŠŅŃ³äÓŠNO2ĘųĢ壩½ųŠŠŹµŃ飮æɹŪ²ģµ½BĘæÖŠĘųĢåŃÕÉ«±ČAĘæÖŠµÄÉī£¬ĘäŌŅņŹĒ_____________”£
”¾“š°ø”æ
£Ø1£©TiO2xH2O”¢Fe£»
£Ø2£©1:4£»
£Ø3£©·ĄÖ¹NH4HCO3·Ö½ā£Ø»ņ¼õÉŁFe3+Ė®½ā£©
£Ø4£©Fe2++2HCO3-=FeCO3”ż+H2O+CO2”ü£»
£Ø5£©FeCO3£Øs£©![]() Fe2+£Øaq£©+CO32-£Øaq£©£¬CO32-ÓėČéĖį·“Ó¦ÅØ¶Č½µµĶ£¬Ę½ŗāĻņÓŅŅĘ¶Æ£¬Ź¹Ģ¼ĖįŃĒĢśČܽāµĆµ½ČéĖįŃĒĢśČÜŅŗ£»
Fe2+£Øaq£©+CO32-£Øaq£©£¬CO32-ÓėČéĖį·“Ó¦ÅØ¶Č½µµĶ£¬Ę½ŗāĻņÓŅŅĘ¶Æ£¬Ź¹Ģ¼ĖįŃĒĢśČܽāµĆµ½ČéĖįŃĒĢśČÜŅŗ£»
£Ø6£©2SO42”Ŗ+2e-=S2O82-
£Ø7£©H2O2·Ö½ā·ÅČČ£¬Ź¹Ę½ŗā2NO2![]() N2O4ĻņÉś³ÉNO2·½ĻņŅĘ¶Æ£»
N2O4ĻņÉś³ÉNO2·½ĻņŅĘ¶Æ£»
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ·ĻŅŗÖŠŗ¬ÓŠ“óĮæFeSO4”¢H2SO4ŗĶÉŁĮæFe2£ØSO4£©3”¢TiOSO4£¬¼ÓĢśŠ¼£¬FeÓėH2SO4ŗĶÉŁĮæFe2£ØSO4£©3·“Ӧɜ³ÉFeSO4£¬TiOSO4Ė®½āÉś³ÉTiO2xH2O£¬¹żĀĖ£¬ĀĖŌüĪŖTiO2xH2O”¢Fe£¬ĀĖŅŗĪŖFeSO4£¬FeSO4ČÜŅŗĶعżÕō·¢ÅØĖõ”¢ĄäČ“½į¾§”¢¹żĀĖĻ“µÓµĆµ½ĮņĖįŃĒĢś¾§Ģ壬ĶŃĖ®”¢ģŃÉÕµĆµ½Ńõ»ÆĢś£»FeSO4ČÜŅŗÖŠ¼ÓĢ¼ĖįĒāļ§Ģ¼ĖįŃĒĢś³Įµķ”¢ĮņĖįļ§ŗĶ¶žŃõ»ÆĢ¼£¬Ģ¼ĖįŃĒĢś³Įµķ¼ÓČéĖįČܽāÉś³ÉČéĖįŃĒĢśČÜŅŗŗĶ¶žŃõ»ÆĢ¼£¬ČéĖįŃĒĢśČÜŅŗĶعżÕō·¢ÅØĖõ”¢ĄäČ“½į¾§”¢¹żĀĖĻ“µÓµĆµ½ČéĖįŃĒĢś¾§Ģ唣
£Ø1£©ÓÉĮ÷³Ģ·ÖĪöæÉÖŖ£¬²½Öč¢ŁĖłµĆĀĖŌüµÄÖ÷ŅŖ³É·ÖĪŖTiO2xH2O”¢Fe£»¹Ź“š°øĪŖ£ŗTiO2xH2O”¢Fe£»
£Ø2£©ĮņĖįŃĒĢśŌŚæÕĘųÖŠģŃÉÕÉś³ÉĢśŗģŗĶČżŃõ»ÆĮņµÄ·½³ĢŹ½ĪŖ£ŗ4FeSO4+O2![]() 2Fe2O3+4SO3£¬Ńõ»Æ¼ĮŹĒŃõĘų£¬»¹Ō¼ĮŹĒŃõ»ÆĢś£¬ĖłŅŌŃõ»Æ¼ĮŗĶ»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ4£¬¹Ź“š°øĪŖ£ŗ1£ŗ4£»
2Fe2O3+4SO3£¬Ńõ»Æ¼ĮŹĒŃõĘų£¬»¹Ō¼ĮŹĒŃõ»ÆĢś£¬ĖłŅŌŃõ»Æ¼ĮŗĶ»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ4£¬¹Ź“š°øĪŖ£ŗ1£ŗ4£»
£Ø3£©Ģ¼ĖįĒāļ§ŹÜČČČŻŅ×·Ö½ā£¬²½Öč¢ÜæŲÖĘ·“Ó¦ĪĀ¶ČµĶÓŚ35”ęµÄÄæµÄŹĒ·ĄÖ¹NH4HCO3·Ö½ā£¬¹Ź“š°øĪŖ£ŗ·ĄÖ¹NH4HCO3·Ö½ā£»
£Ø4£©ÓÉĮ÷³ĢĶ¼æÉÖŖ£¬ĮņĖįŃĒĢśÓėĢ¼ĖįĒāļ§·“Ó¦ŹĒĢ¼ĖįŃĒĢś£¬»¹Éś³ÉĘųĢåĪŖ¶žŃõ»ÆĢ¼£¬ČÜŅŗBĪŖĮņĖįļ§ČÜŅŗ£¬·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖ£ŗFe2++2HCO3-=FeCO3”ż+H2O+CO2”ü£¬¹Ź“š°øĪŖ£ŗFe2++2HCO3-=FeCO3”ż+H2O+CO2”ü£»
£Ø5£©Ģ¼ĖįŃĒĢśµÄ³Įµķ“ęŌŚČܽāĘ½ŗā£ŗFeCO3£Øs£©![]() Fe2+£Øaq£©+CO32-£Øaq£©£¬¼ÓČėČéĖį£¬ÕāŃłCO32-ÓėČéĖį·“Ó¦ÅØ¶Č½µµĶ£¬Ę½ŗāĻņÓŅŅĘ¶Æ£¬Ź¹Ģ¼ĖįŃĒĢśČܽāµĆµ½ČéĖįŃĒĢśČÜŅŗ£¬¹Ź“š°øĪŖ£ŗFeCO3£Øs£©
Fe2+£Øaq£©+CO32-£Øaq£©£¬¼ÓČėČéĖį£¬ÕāŃłCO32-ÓėČéĖį·“Ó¦ÅØ¶Č½µµĶ£¬Ę½ŗāĻņÓŅŅĘ¶Æ£¬Ź¹Ģ¼ĖįŃĒĢśČܽāµĆµ½ČéĖįŃĒĢśČÜŅŗ£¬¹Ź“š°øĪŖ£ŗFeCO3£Øs£©![]() Fe2+£Øaq£©+CO32-£Øaq£©£¬CO32-ÓėČéĖį·“Ó¦ÅØ¶Č½µµĶ£¬Ę½ŗāĻņÓŅŅĘ¶Æ£¬Ź¹Ģ¼ĖįŃĒĢśČܽāµĆµ½ČéĖįŃĒĢśČÜŅŗ£»
Fe2+£Øaq£©+CO32-£Øaq£©£¬CO32-ÓėČéĖį·“Ó¦ÅØ¶Č½µµĶ£¬Ę½ŗāĻņÓŅŅĘ¶Æ£¬Ź¹Ģ¼ĖįŃĒĢśČܽāµĆµ½ČéĖįŃĒĢśČÜŅŗ£»
£Ø6£©µē½āŹ±£¬Ńō¼«·¢ÉśŃõ»Æ·“Ó¦£¬ŃĒĮņĖįøłĄė×ÓŹ§Č„µē×ÓÉś³ÉS2O82-£¬µē¼«·“Ó¦ĪŖ2SO42”Ŗ+2e-=S2O82-£¬¹Ź“š°øĪŖ£ŗ2SO42”Ŗ+2e-=S2O82-£»
£Ø7£©ÓÉĶ¼aæÉÖŖ£¬1mol¹żŃõ»ÆĒā×ÜÄÜĮæøßÓŚ1molĖ®Óė0.5molŃõĘų×ÜÄÜĮ棬¹Ź¹żŃõ»ÆĒā·Ö½āŹĒ·ÅČČ·“Ó¦£¬ÓÉĶ¼bæÉÖŖ£¬2mol¶žŃõ»ÆµŖµÄÄÜĮæøßÓŚ1molĖÄŃõ»Æ¶žµŖµÄÄÜĮ棬¹Ź¶žŃõ»ÆµŖ×Ŗ»ÆĪŖĖÄŃõ»Æ¶žµŖµÄ·“Ó¦ĪŖ·ÅČČ·“Ó¦£¬ĖłŅŌĶ¼cÖŠ£¬ÓŅ²ąÉÕ±µÄĪĀ¶ČøßÓŚ×ó²ą£¬ÉżøßĪĀ¶ČŹ¹2NO2£Øŗģ×ŲÉ«£©![]() N2O4£ØĪŽÉ«£©”÷H£¼0£¬ĻņÄę·“Ó¦·½ĻņŅĘ¶Æ£¬¼“ĻņÉś³ÉNO2ŅĘ¶Æ£¬¹ŹBĘæŃÕÉ«øüÉī£¬¹Ź“š°øĪŖ£ŗH2O2·Ö½ā·ÅČČ£¬Ź¹Ę½ŗā2NO2
N2O4£ØĪŽÉ«£©”÷H£¼0£¬ĻņÄę·“Ó¦·½ĻņŅĘ¶Æ£¬¼“ĻņÉś³ÉNO2ŅĘ¶Æ£¬¹ŹBĘæŃÕÉ«øüÉī£¬¹Ź“š°øĪŖ£ŗH2O2·Ö½ā·ÅČČ£¬Ź¹Ę½ŗā2NO2![]() N2O4ĻņÉś³ÉNO2·½ĻņŅĘ¶Æ”£
N2O4ĻņÉś³ÉNO2·½ĻņŅĘ¶Æ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”涞Ńõ»ÆĮņ”¢µŖĘų”¢¶žŃõ»ÆĢ¼×é³ÉµÄ»ģŗĻĘųĢåŌŚĶ¬ĪĀ”¢Ķ¬Ń¹ĻĀÓėŠ¦Ęų£ØN2O£©µÄĆܶČĻąĶ¬£¬ŌņøĆ»ģŗĻĘųĢåÖŠ¶žŃõ»ÆĮņ”¢µŖĘų”¢¶žŃõ»ÆĢ¼µÄÖŹĮæ±ČĪŖ£Ø £©
A.4£ŗ5£ŗ6B.22£ŗ1£ŗ14
C.64£ŗ35£ŗ13D.29£ŗ8£ŗ13
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻņFe2+”¢I-”¢Br-µÄČÜŅŗÖŠĶØČėŹŹĮæCl2,ČÜŅŗÖŠø÷ÖÖĄė×ÓµÄĪļÖŹµÄĮæ±ä»ÆČēĶ¼ĖłŹ¾”£ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ( )
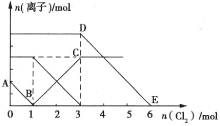
A.Ļ߶ĪBC“ś±ķCl-ĪļÖŹµÄĮæµÄ±ä»ÆĒéæö
B.Ō»ģŗĻČÜŅŗÖŠc(FeBr2)=6mol”¤L-1
C.µ±ĶØČė2mol Cl2Ź±,ČÜŅŗÖŠŅŃ·¢ÉśµÄĄė×Ó·“Ó¦æɱķŹ¾ĪŖ2Fe2++2I-+2Cl2=2Fe3++I2+4Cl-
D.ŌČÜŅŗÖŠn(Fe2+):n(I-):n(Br-)=2:2:3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅŃÖŖH2C2O4ŹĒ¶žŌŖČõĖį£¬ŹŅĪĀĻĀĻņijÅØ¶ČµÄ²ŻĖįČÜŅŗÖŠÖšµĪ¼ÓČėKOHČÜŅŗ£¬ĖłµĆČÜŅŗÖŠH2C2O4”¢HC2O4-”¢C2O42-µÄ×é³É°Ł·ÖĀŹÓėpHµÄ¹ŲĻµČēĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
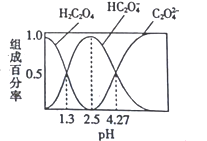
A. pH=4.27µÄČÜŅŗÖŠ£ŗc(K+)+c(H+)=c(OH-)+3c(C2O42-)
B. ·“Ó¦HC2O4-+H2O![]() H2C2O4+OH-µÄĘ½ŗā³£ŹżĪŖ10-4.27
H2C2O4+OH-µÄĘ½ŗā³£ŹżĪŖ10-4.27
C. ĻņČÜŅŗÖŠ¼ÓČėKOHČÜŅŗ½«pHÓÉ1.3µ÷ÖĮ4.27µÄ¹ż³ĢÖŠĖ®µÄµēĄė³Ģ¶ČĻČŌö“óŗó¼õŠ”
D. pH=2.5µÄČÜŅŗÖŠ£ŗc(H+)+2c(H2C2O4)=c(OH-)+c(C2O42-)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ”°84Ļū¶¾Ņŗ”±ÄÜÓŠŠ§É±Ćš¼×ŠĶH1N1µČ²”¶¾£¬Ä³Ķ¬Ń§źĄĀņĮĖ Ņ»Ęæ”°84Ļū¶¾Ņŗ”±£¬²¢ĖŌÄĻą¹Ų׏ĮĻŗĶĻū¶¾Ņŗ°ü×°ĖµĆ÷µĆµ½ ČēĻĀŠÅĻ¢£ŗ”°84Ļū¶¾Ņŗ”±ŗ¬25%NaC10”¢1000 mL”¢ĆÜ¶Č 1. 192 g/cm 3,Ļ”ŹĶ100±¶(Ģå»ż±Č)ŗóŹ¹ÓĆ”£Ēėøł¾ŻŅŌÉĻŠÅĻ¢ŗĶĻą¹ŲÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©øĆ”°84Ļū¶¾Ņŗ”±ÖŠNaClOµÄĪļÖŹµÄĮæÅضČĪŖ________ mol/L”£
£Ø2£©øĆĶ¬Ń§Č”100 mLĻ”ŹĶŗóµÄĻū¶¾ŅŗÓĆÓŚĻū¶¾£¬Ļ”ŹĶŗóČÜŅŗÖŠ c(Na+)ĪŖ_______mol/L”£
£Ø3£©øĆĶ¬Ń§²ĪŌÄøĆ”°84Ļū¶¾Ņŗ”±µÄÅä·½£¬ÓūÓĆNaClO¹ĢĢåÅäÖĘ480 mLŗ¬4. 0 mol/L NaClOµÄĻū¶¾Ņŗ”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ________£ØĢīŠņŗÅ)”£
A.ČŻĮæĘæÓĆÕōĮóĖ®Ļ“¾»ŗó£¬Ó¦ŗęøÉŗó²ÅÄÜÓĆÓŚČÜŅŗÅäÖĘ
B.ĄūÓĆ¹ŗĀņµÄÉĢĘ·NaClOĄ“ÅäÖĘ£¬æÉÄܵ¼ÖĀ½į¹ūĘ«µĶ
C.ŠčŅŖ³ĘĮæNaClO¹ĢĢåµÄÖŹĮæĪŖ143. 0 g
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ·¼ĻćĢžAŹĒ»ł±¾ÓŠ»ś»Æ¹¤ŌĮĻ£¬ÓÉAÖʱøøß·Ö×ÓEŗĶŅ½Ņ©ÖŠ¼äĢåKµÄŗĻ³ÉĀ·Ļߣزæ·Ö·“Ó¦Ģõ¼žĀŌČ„£©ČēĻĀĶ¼ĖłŹ¾£ŗ
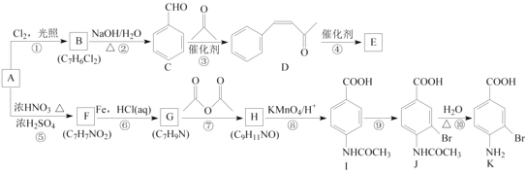
¼ŗÖŖ£ŗ¢Ł![]() £»
£»
¢Ś
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AµÄĆū³ĘŹĒ________£¬Iŗ¬ÓŠ¹ŁÄÜĶŵÄĆū³ĘŹĒ________”£
£Ø2£©·“Ó¦¢ßµÄ×÷ÓĆŹĒ________£¬¢āµÄ·“Ó¦ĄąŠĶŹĒ________”£
£Ø3£©Š“³ö·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½£ŗ____________________________________________________”£
£Ø4£©D·Ö×ÓÖŠ×ī¶ąÓŠ________øöŌ×Ó¹²Ę½Ćę”£EµÄ½į¹¹¼ņŹ½ĪŖ________”£
£Ø5£©Š“³öŅ»ÖÖĶ¬Ź±·ūŗĻĻĀĮŠĢõ¼žµÄFµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ________”£
¢Ł±½»·ÉĻÖ»ÓŠĮ½ÖÖ²»Ķ¬»Æѧ»·¾³µÄĒāŌ×Ó£»
¢Ś¼ČÄÜÓėŅų°±ČÜŅŗ·“Ó¦ÓÖÄÜÓėNaOHČÜŅŗ·“Ó¦”£
£Ø6£©½«ÓÉDĪŖĘšŹ¼ŌĮĻÖʱø![]() µÄŗĻ³ÉĀ·Ļß²¹³äĶźÕū”£
µÄŗĻ³ÉĀ·Ļß²¹³äĶźÕū”£
 ________£ØĪŽ»śŹŌ¼Į¼°ČܼĮČĪŃ”£©”£
________£ØĪŽ»śŹŌ¼Į¼°ČܼĮČĪŃ”£©”£
ŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼Ź¾ĄżČēĻĀ£ŗ
CH3CHO![]() CH3COOH
CH3COOH![]() CH3COOCH2CH3
CH3COOCH2CH3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ°×Į×ÓėŃõæÉ·¢ÉśČēĻĀ·“Ó¦£ŗP4+5O2=P4O10”£ŅŃÖŖ¶ĻĮŃĻĀĮŠ»Æѧ¼üŠčŅŖĪüŹÕµÄÄÜĮæ·Ö±šĪŖ£ŗP”ŖP akJ”¤mol”Ŗ1”¢P”ŖO bkJ”¤mol”Ŗ1”¢P="O" ckJ”¤mol”Ŗ1”¢O="O " dkJ”¤mol”Ŗ1”£

øł¾ŻĶ¼Ź¾µÄ·Ö×Ó½į¹¹ŗĶÓŠ¹ŲŹż¾Ż¹ĄĖćøĆ·“Ó¦µÄ”÷H£¬ĘäÖŠÕżČ·µÄŹĒ£Ø £©
A. £Ø6a+5d£4c£12b£©kJ”¤mol”Ŗ1B£Ø4c+12b£6a£5d£©kJ”¤mol”Ŗ1 B. £Ø4c+12b£4a£5d£©kJ”¤mol”Ŗ1
C. £Ø4a+5d£4c£12b£©kJ”¤mol”Ŗ1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æøĒĖ¹¶ØĀÉČĻĪŖÄÜĮæ×ÜŹĒŹŲŗćµÄ£ŗ»Æѧ·“Ó¦¹ż³ĢŅ»²½Ķź³É»ņ·Ö²½Ķź³É£¬Õūøö¹ż³ĢµÄČČŠ§Ó¦ŹĒĻąĶ¬µÄ”£
ŅŃÖŖ£ŗ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
ČōŹ¹23g¾Ę¾«ŅŗĢåĶźČ«Č¼ÉÕ£¬×īŗó»Öø“µ½ŹŅĪĀ£¬Ōņ·Å³öµÄČČĮæĪŖ£Ø £©
A.![]() B.
B.![]()
C.![]() D.
D.![]()
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅŃÖŖSO2£«I2£«2H2O===H2SO4£«2HI£¬Ä³»ÆѧŠĖȤŠ”×éŃ”ÓĆĻĀĮŠŹµŃé×°ÖĆ£¬²ā¶Ø¹¤ŅµŌĮĻĘų(ŗ¬SO2”¢N2”¢O2)ÖŠSO2µÄŗ¬Į攣

(1)ČōŌĮĻĘų“Ó×óĮ÷ĻņÓŅŹ±£¬ÉĻŹö×°ÖĆ×é×°Į¬½ÓµÄĖ³Šņ£ŗŌĮĻĘų”ś__________________(Ģī”°a”±”°b”±”°c”±”°d”±”°e”±)”£
(2)×°ÖĆ¢ņÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_____________£»µ±×°ÖĆ¢ņÖŠ³öĻÖ________ĻÖĻóŹ±£¬Į¢¼“Ķ£Ö¹ĶØĘų”£
(3)ČōµāČÜŅŗµÄÅضČĪŖ0.05 mol/L”¢Ģå»żĪŖ20 mL£¬ŹÕ¼Æµ½µÄN2ÓėO2µÄĢå»żĪŖ297.6 mL(ŅŃÕŪĖćĪŖ±ź×¼×“æöĻĀµÄĢå»ż)£¬SO2µÄĢå»ż·ÖŹżĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com