
��9�֣� ���ᣨHCOOH����һ���д̼���ζ����ɫҺ�壬�к�ǿ�ĸ�ʴ�ԡ��۵�8.4�棬�е�100.7�棬����ˮ���Ҵ����ܣ�������160�漴�ֽ�ɶ�����̼��������
��1��ʵ���ҿ��ü�����Ũ���Ṳ���Ʊ�һ����̼��HCOOHŨ����========H2O+CO����ʵ��IJ���װ������ͼ��ʾ���Ʊ�ʱ�ȼ���Ũ������80�桪90�棬����ε�����ᡣ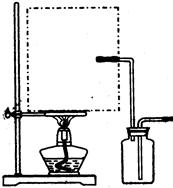

���Ʊ�CO �� ���ռ�CO
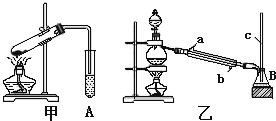
�ٴ���ͼ��ѡ���������������������ȱ�����巢��װ�ã����ӱ�Ҫ�����ӡ������ܡ���Ƥ�ܣ��̶�װ�ò��û����������������е��Լ�����2�֣�


��Һ©�� ����©�� ������ƿ ����ƿ �¶ȼ�
�� װ�â�������� ����2�֣�
��2��ʵ���ҿ��ü����Ʊ�����ͭ���䷽������������ͭ��̼�����������Ƶü�ʽ̼��ͭ��Ȼ��������ᷴӦ�Ƶ���ˮ����ͭ[Cu(HCOO)2��4H2O]���塣��صĻ�ѧ����ʽ�ǣ�
2CuSO4+4 NaHCO3= Cu(OH)2��CuCO3��+3CO2��+2Na2SO4+H2O
Cu(OH)2��CuCO3+4HCOOH+ 5H2O="2" Cu(HCOO)2��4H2O+ CO2��
ʵ�鲽�����£�
��ʽ̼��ͭ���Ʊ���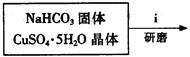
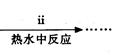
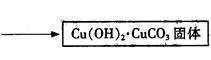
�۲��袡�ǽ�һ����CuSO4��5H2O�����NaHCO3����һ��ŵ��в�����ĥ����Ŀ���� ����2�֣�
�ܲ��袢���ڽ����½���������ֶ�λ���������ˮ�У���Ӧ�¶ȿ�����70�桪80�棬������� ����дʵ������˵���¶ȹ��ߡ���1�֣�
����ͭ���Ʊ���
��Cu��OH��2��CuCO3��������ձ��У�����һ�����ȵ�����ˮ������μ����������ʽ̼��ͭǡ��ȫ���ܽ⣬���ȹ��˳�ȥ�������������ʡ���ͨ�����������Һ��ԭ�����1/3ʱ����ȴ�������壬���ˣ�����������ˮ�Ҵ�ϴ�Ӿ���2��3�Σ����ɣ��õ���Ʒ��
�ݡ����ȹ��ˡ��У����롰���ȡ���ԭ���� ����1�֣�
�����Ҵ�ϴ�Ӿ����Ŀ���� ����1�֣�
 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| ���� |
| Ũ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ŨH2SO4 |
| ���� |
| ŨH2SO4 |
| ���� |
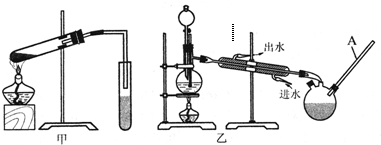
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| [HCOO-] | [HCOOH]?[OH-] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
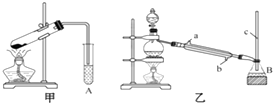 ij��ѧ��ȤС���üס�������װ�ã���ͼ��ʾ�����м��ᣨHCOOH����״���CH3OH��������Ӧ��ʵ�飬�ش��������⣺
ij��ѧ��ȤС���üס�������װ�ã���ͼ��ʾ�����м��ᣨHCOOH����״���CH3OH��������Ӧ��ʵ�飬�ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com