
 ��Уij��ѧС�������ʵ�����ŨH2SO4��ľ̿��Ӧ�����в��
��Уij��ѧС�������ʵ�����ŨH2SO4��ľ̿��Ӧ�����в��| ѡ�õ����� ������ĸ�� | ������Լ� | ���� |
| ��1��______ | ��ˮ����ͭ | ______ |
| ��2��A | ||
| ��3��______ | ����KMnO4��Һ | ______ |
| ��4��______ | ______ | ����SO2�Ƿ��ѳ��� |
| ��5��A | ______ | ______ |
 2S02��+C02��+2H2O���ʴ�Ϊ��C+2H2SO4��Ũ��
2S02��+C02��+2H2O���ʴ�Ϊ��C+2H2SO4��Ũ��  2S02��+C02��+2H2O��
2S02��+C02��+2H2O��| ѡ�õ����� ������ĸ�� | ������Լ� | ���� |
| ��1��B ��2��A ��3��A ��4��A ��5��A | ��ˮ����ͭ Ʒ�� ����KMnO4��Һ Ʒ�� ����ʯ��ˮ | ����ˮ������ ����SO2������ ��ȥSO2 ����SO2�Ƿ��ѳ��� ����CO2������ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Уij��ѧС�������ʵ�����ŨH2SO4��ľ̿��Ӧ�����в��
��Уij��ѧС�������ʵ�����ŨH2SO4��ľ̿��Ӧ�����в��
| ||
| ||
| ѡ�õ����� ������ĸ�� |
������Լ� | ���� |
| ��1�� B B |
��ˮ����ͭ | ����ˮ������ ����ˮ������ |
| ��2��A | ||
| ��3�� A A |
����KMnO4��Һ | ��ȥSO2 ��ȥSO2 |
| ��4�� A A |
Ʒ�� Ʒ�� |
����SO2�Ƿ��ѳ��� |
| ��5��A | ����ʯ��ˮ ����ʯ��ˮ |
����CO2������ ����CO2������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Уij��ѧС�������ʵ�����ŨH2SO4��ľ̿��Ӧ�����в���:
(1)д��ŨH2SO4��ľ̿��Ӧ�Ļ�ѧ����ʽ
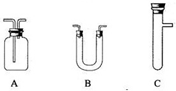
(2)�������ͼ��ѡ����������������ظ�ʹ�ã����һ��װ�ð�����С��ʵ�����ǵ�Ŀ��.���ṩŨH2SO4��ľ̿������KMnO4��Һ�������̡�Һ�Լ���ѡ�������Ӻ̶������õIJ����ܡ����ܡ����С�����̨������װ�õȾ���ȥ��
����ѡ������������˳�������������������±�(���Բ�����,Ҳ���Բ���)����д����������Ӧ���Լ������Ƽ������á�
| ѡ�õ����� ������ĸ�� | ������Լ� | ���� |
| (1) (2) (3) (4) (5) �� �� �� |
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012����Ϻ�����У��һ��ѧ����ĩ������⻯ѧ�Ծ����������� ���ͣ�ʵ����
��Уij��ѧС�������ʵ�����ŨH2SO4��ľ̿��Ӧ�����в���:
(1)д��ŨH2SO4��ľ̿��Ӧ�Ļ�ѧ����ʽ
(2)�������ͼ��ѡ����������������ظ�ʹ�ã����һ��װ�ð�����С��ʵ�����ǵ�Ŀ��.���ṩŨH2SO4��ľ̿������KMnO4��Һ����ˮ����ͭ�������̡�Һ�Լ���ѡ�������Ӻ̶������õIJ����ܡ����ܡ����С�����̨������װ�õȾ���ȥ��
����ѡ������������˳�������������������±�(���Բ�����,Ҳ���Բ���)����д����������Ӧ���Լ������Ƽ������á�
| ѡ�õ����� ������ĸ�� | ������Լ� | ���� |
| (1) | ��ˮ����ͭ | |
| (2) A | | |
| (3) | ����KMnO4��Һ | |
| (4) | | ����SO2�Ƿ��ѳ��� |
| (5) A | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012����Ϻ�����У��һ��ѧ����ĩ������⻯ѧ�Ծ��������棩 ���ͣ�ʵ����
��Уij��ѧС�������ʵ�����ŨH2SO4��ľ̿��Ӧ�����в���:
(1)д��ŨH2SO4��ľ̿��Ӧ�Ļ�ѧ����ʽ
(2)�������ͼ��ѡ����������������ظ�ʹ�ã����һ��װ�ð�����С��ʵ�����ǵ�Ŀ��.���ṩŨH2SO4��ľ̿������KMnO4��Һ����ˮ����ͭ�������̡�Һ�Լ���ѡ�������Ӻ̶������õIJ����ܡ����ܡ����С�����̨������װ�õȾ���ȥ��

����ѡ������������˳�������������������±�(���Բ�����,Ҳ���Բ���)����д����������Ӧ���Լ������Ƽ������á�
|
ѡ�õ����� ������ĸ�� |
������Լ� |
���� |
|
(1) |
��ˮ����ͭ |
|
|
(2) A |
|
|
|
(3) |
����KMnO4��Һ |
|
|
(4) |
|
����SO2�Ƿ��ѳ��� |
|
(5) A |
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��Уij��ѧС�������ʵ�����ŨH2SO4��ľ̿��Ӧ�����в���:
(1)д��ŨH2SO4��ľ̿��Ӧ�Ļ�ѧ����ʽ
(2)�������ͼ��ѡ����������������ظ�ʹ�ã����һ��װ�ð�����С��ʵ�����ǵ�Ŀ��.���ṩŨH2SO4��ľ̿������KMnO4��Һ�������̡�Һ�Լ���ѡ�������Ӻ̶������õIJ����ܡ����ܡ����С�����̨������װ�õȾ���ȥ��

����ѡ������������˳�������������������±�(���Բ�����,Ҳ���Բ���)����д����������Ӧ���Լ������Ƽ������á�
|
ѡ�õ����� ������ĸ�� |
������Լ� |
���� |
|
(1) (2) (3) (4) (5) �� �� �� |
|
|
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com