
| 5 |
| 2 |
| 3.2 |
| at |
| 3.2 |
| at |


 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | ��A | ��A | �� | ��A | ��A | ��A | ��A | 0 |
| 1 | a | |||||||
| 2 | b | c | d | e | ||||
| 3 | f | g | h | i | j | k | m | n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
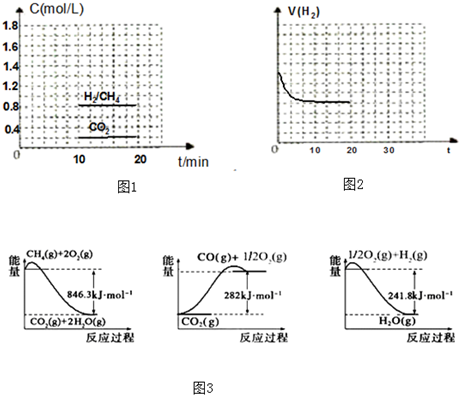
 2SO2��g��+O2��g��=2SO3��g����H=-198kJ?mol-1��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
2SO2��g��+O2��g��=2SO3��g����H=-198kJ?mol-1��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
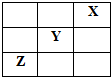 X��Y��Z�Ǣ�����A������ַǽ���Ԫ�أ����������ڱ��е�λ����ͼ��ʾ���Իش�
X��Y��Z�Ǣ�����A������ַǽ���Ԫ�أ����������ڱ��е�λ����ͼ��ʾ���Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
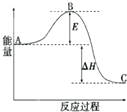
| A����101kPaʱ��1mol������ȫȼ��ʱ���ų������������������ʵ�ȼ���� |
| B����ͼ���кͷ�Ӧ����1molˮ����ʱ�ķ�Ӧ�Ƚ��к��� |
| C�������ʵ������������������ֱ���ȫȼ�գ����߷ų��������� |
| D���ɵ���Aת��Ϊ����B����H=+119KJ/mol����֪����A�ȵ���B�ȶ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com