
X��Y��Z��Ϊ������Ԫ�أ�ԭ��������������X�ĵ���Ϊ�ܶ���С�����壬Yԭ������������������������������Z��Xԭ������������ͬ���ش��������⣺
��1��X��Y��Z��Ԫ�ط��ŷֱ�Ϊ �� �� ��
��2��������Ԫ����ɵĻ������У��Ⱥ��й��ۼ��ֺ������Ӽ����� �� ��
��3��X��Y��ɵĻ������У��Ⱥ��м��Թ��ۼ��ֺ��зǼ��Թ��ۼ����� ���˻������������������������ط�Ӧ�����ӷ���ʽΪ ���˻����ﻹ�ɽ����Թ�ҵ��ˮ�е�CN-����Ϊ̼���κͰ�����Ӧ�����ӷ���ʽΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣��±�ΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã����û�ѧ����
�ش��������⣺
��1���ܡ��ݡ��ߵ�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ_______����Ԫ�ط��ű�ʾ����ͬ����
��2���͢ߵ���ۺ����������ǿ��Ϊ_______>_______��
��3���١�������Ԫ�ذ�ԭ�Ӹ���֮��Ϊ1��1��ɵij���Һ̬�������������Һ���ܽ�Fe��������д���÷�Ӧ�����ӷ���ʽ_______��
��4���ɱ���Ԫ���γɵ����ʿɷ�����ͼ�еķ�Ӧ��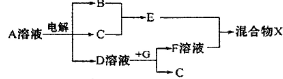
����B��C��G�ǵ��ʣ�BΪ����ɫ���壬D��Һ�Լ��ԡ�
��д��D��Һ��G��Ӧ�Ļ�ѧ����ʽ______________.
��д������A��Һ�����ʵ������ӵķ�����______________.
�۳����£������1 L 0.1 mol/L��A��Һ��һ��ʱ�������ҺpHΪ12��������Һ����仯������õ�������ת�Ƶ��ӵ����ʵ���Ϊ_______mol��
������ͼ�и�����Ӧ��Ϊǡ����ȫת����������X�к��е�������_______ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��13�֣�������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������T��������������������������ȣ���ش��������⣺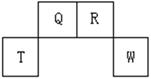
(1)W�����ڱ��е�λ���� �� Q��R��T����Ԫ��ԭ�ӵİ뾶�Ӵ�С����˳�� ����Ԫ�ط��ű�ʾ����Q�����������ĵ���ʽ ��R��̬�⻯����ӵĽṹʽΪ ��
(2)Ԫ�ص�ԭ�ӵõ���������Q W���ǿ�ڡ������ڡ�����
(3)ԭ��������R��8��Ԫ���γɵ�һ�ֳ�����̬�⻯��ķе� ����ߡ��͡�����R�ĵij�����̬�⻯�
(4)T��Q��R��W�ĵ����У���̬ʱ����ԭ�Ӿ������ �������ƣ���
(5)����8��Ԫ�ص����ʡ��������±����У��������ڶ����ڣ���ָ��RԪ�����±��еĶ�Ӧ��� ����Tͬ����������������ˮ���������ǿ��Ԫ�����±��еĶ�Ӧ��� ��
| | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.6 | 0.75 | 0.82 |
| �����ͻ��ϼ� | | +2 | +1 | +5 | +7 | +1 | +5 | +3 |
| -2 | | | -3 | -1 | | -3 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����A��B��C��D���ֶ���������Ԫ�أ�AԪ���γɵġ�2�������ӱȺ�ԭ�ӵĺ����������8����BԪ����AԪ���γɵ�һ�ֻ�����Ϊ����ɫ���壬�ù�����������������A�ĵ��ʣ�CΪԭ�Ӻ�����12�����ӵĽ���Ԫ�أ���2.4��C��������ˮ��Ӧʱ���ڱ�״���·ų�����2.24L��C�γ������������ӣ�DԪ��ԭ�ӵ�M������7�����ӡ�
��1��д��B��C����Ԫ�ص�Ԫ�ط��ţ�B ��C ��
��2������A2-�����ӽṹʾ��ͼ�� �� ָ��D�����ڱ���λ�ã� ��
��3��д��B�ijʵ���ɫ�Ļ�������CO2��Ӧ�Ļ�ѧ����ʽ�� ��
��4���Ƚ�D����̬�⻯����H2S��HF���ȶ�����ǿ������˳�� ���û�ѧʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��W��M��N����Ԫ�طֱ�λ�����ڱ����������ڵ����ڣ���ԭ������������X��Y���⻯�ﶼ��ͬ��Ԫ���⻯��ķе�ߣ�����ͬ������ȴ������ߵġ�W��ͬ����Ԫ�������Ӱ뾶��С��Ԫ�ء�Mԭ�ӵ������ܲ����������˶�״̬��ͬ�ĵ��ӡ�N��һ�֡����ǽ��������㷺Ӧ���ں��졢���µȹ�ҵ������ش��������⣺
��X��Y����Ԫ�ص�Ԫ�ط����ǣ� �� ��X��Y�����γ�һ�ֹ��ۻ������������Ԫ���������������ﵽ8��������ӵĿռ乹���ǣ� ������ԭ�ӵ��ӻ���ʽ�ǣ� ��
��X���⻯��������ˮ����ԭ���ǣ� ��
��N�ĵ����Ų�ʽ�ǣ� ��
��X��Y��Ԫ�صĵ�һ�����ܴ�С��ϵ�� С�� (��Ԫ�ط���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z��W��R��Ԫ�����ڱ�ǰ�������еij���Ԫ��,�������Ϣ���±�:
| Ԫ�� | �����Ϣ |
| X | ��ɵ����ʵĻ���Ԫ��,����������ϼ���������ϼ۵Ĵ�����Ϊ2 |
| Y | �ؿ��к�����ߵ�Ԫ�� |
| Z | ����������Ϊ23,������Ϊ11�ĺ��� |
| W | �����д���ʹ����Ͻ���Ʒ,��ҵ�Ͽ��õ������������ķ����Ʊ��䵥�� |
| R | �ж��ֻ��ϼ�,���ɫ���������ڿ����л�Ѹ�ٱ�ɻ���ɫ,����ɺ��ɫ |
 W�Ͻ�(Z17W12)��һ��DZ�ڵ��������,��Z��W������һ���������������ɡ��úϽ���һ����������ȫ����ķ�Ӧ����ʽΪ:Z17W12+17H2
W�Ͻ�(Z17W12)��һ��DZ�ڵ��������,��Z��W������һ���������������ɡ��úϽ���һ����������ȫ����ķ�Ӧ����ʽΪ:Z17W12+17H2 17ZH2+12W,�õ��Ļ����Q(17ZH2+12W)��6.0 mol��L-1HCl��Һ������ȫ�ͷų�H2��1 mol Z17W12��ȫ�����õ��Ļ����Q������������ȫ��Ӧ,�ͷų�H2�����ʵ���Ϊ ��
17ZH2+12W,�õ��Ļ����Q(17ZH2+12W)��6.0 mol��L-1HCl��Һ������ȫ�ͷų�H2��1 mol Z17W12��ȫ�����õ��Ļ����Q������������ȫ��Ӧ,�ͷų�H2�����ʵ���Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��32Geԭ�Ӻ�������� �ֲ�ͬ���˶�״̬��ԭ�Ӻ�������Ų�ʽΪ ��
��2��д����ԭ�ӵĺ�����ӵĹ����ʾʽ ��
��3��д��CCl4�ĵ���ʽ ��������ԭ�ӵ��ӻ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣���X��Y��Z���ֶ�����Ԫ�أ�X����̬�⻯�ﻯѧʽΪH2X�����⻯��ķ�������X���������ķ�����֮��Ϊ17��40��Xԭ�Ӻ�������������������ȣ�Y��X�����γ����ӻ�����Y2X��Y�������ӵ��Ӳ�ṹ��Ne��ͬ��Z��Xͬ���ڣ�����̬������˫ԭ�ӷ��ӣ���ԭ�ӹ���1�Ե��ӡ��Իش�
��1��д����Ԫ�ط��ţ�X ��Y ��Z ��
��2��X���ӵĽṹʾ��ͼΪ ��X��Y�γɵ����ӻ�����ĵ���ʽΪ �� Z�����γɵĻ�����ĵ���ʽΪ ��
��3��Y�����ڿ�����ȼ�յĻ�ѧ����ʽΪ ����������ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F���ֶ���������Ԫ�أ�ԭ��������������
| Ԫ�� | ��Ϣ |
| B | �䵥���ڳ�����Ϊ˫ԭ�ӷ��ӣ���A���γɷ���X��X��ˮ��Һ�ʼ��� |
| D | �����������X������ͬ������������ͬ�����м��������а뾶��С�� |
| E | Ԫ��ԭ�������ȴ������2������ |
| C��F | ����Ԫ�ص�ԭ������㹲��13������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com