

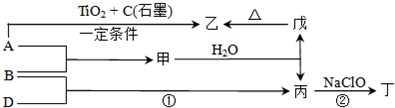
 Na2CO3+CO2��+H2O��
Na2CO3+CO2��+H2O�� Na2CO3+CO2��+H2O��
Na2CO3+CO2��+H2O��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ���¸�ѹ |
| ||
| ���¸�ѹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
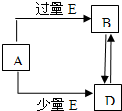 ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� |
| ���¸�ѹ |
| ���� |
| ���¸�ѹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʦ����2006��2007ѧ�����ѧ�ڸ����¿��Ծ�(��)����ѧ���� ���ͣ�022
| |||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
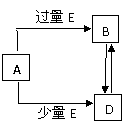 ��08�Ƹ���ѧ��ģ��(15��)��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ(���ֲ�����ȥ)��
��08�Ƹ���ѧ��ģ��(15��)��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ(���ֲ�����ȥ)��
(1) ��A��EΪ���ʣ����A���ʵ�Ԫ������Ȼ�����γɻ�������������Ԫ�ء�
�� B���ڷ��� �Ǽ��� (����ԡ��Ǽ��ԡ�)��B�����и�ԭ������� �� (��ǡ���)������8���ӽṹ��
�� ��50 mL4 mol��L��NaOH��Һ��ͨ��1.12 L B(��״��)��
��Ӧ����Һ�����ʵ����ʵ���֮��Ϊ Na2CO3 :NaOH =1:2 (�ѧʽ�����ʵ���֮��)��
�� 4 g A������ȫȼ�շų�131.2 kJ��������д����ʾA����ȼ���ȵ��Ȼ�ѧ����ʽ
C(s)+O2(g)= CO2(g)����H=393.6kJ/mol
(2) ��AΪ���������е�Ԫ�����γɵ��Ȼ��
�� д��A��Һ��B�����ӷ���ʽ Al3++4OH�D=AlO2�D��2H2O ��
�� д��A��B����Һ��Ӧ�����ӷ���ʽ Al3++3AlO2�D��6H2O=4Al(OH)3�� ��
(3) ��AΪ�����Ľ�������E���䡢Ũ��Һ�У�A�жۻ�������֪��XΪ���зǼ��������ӻ������1 mol X����38 mol���ӣ���D��Һ�м�����D�����ʵ�����X���壬ǡ����ȫ��Ӧ��д���÷�Ӧ�����ӷ���ʽ 4Fe2++4Na2O2��6H2O=4Fe(OH)3��O2����8Na+ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com