
���� ��1����������Ũ��Ϊ0.10mol•L-1��KMnO4����Һ500mL��������Һ���������ʵ���=0.10mol•L-1 ��0.500L=0.0500mol��m=nM����õ�����������
a��������Ʊ���Һ����ѡ�������Ҫ��������������Һ����Ϊ���㡢�������ܽ⡢ת�ơ�ϴ��ת�Ƶ�����ƿ�����ݡ�ҡ�ȵȣ�
b������ʱ���Ӷ�����ˮ���볬���̶��ߣ�
��2��MnO4+Ϊ��������������Һ��MnԪ�ػ��ϼ�+7�۱仯Ϊ+2�ۣ�H2O2 ����ԭ��������Ϊ��������ϵ����غ��ԭ���غ�õ����ӷ���ʽ��
��3�����Ը��������Һ����ǿ�����ԣ��������ܣ��ζ������յ�������ǵ������һ�θ��������Һ����Һ����ɫ�仯Ϊ�Ϻ�ɫ�Ұ���Ӳ��仯��
��4����ϸ��������Һ�������ⷢ����������ԭ��Ӧ������ϵ���㣻
��5�����ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ����ȡ���ı���Һ�����������c�����⣩=$\frac{c������V������}{V�����⣩}$�жϣ�
��� �⣺��1����������Ũ��Ϊ0.10mol•L-1��KMnO4����Һ500mL��������Һ���������ʵ���=0.10mol•L-1 ��0.500L=0.0500mol������=0.0500mol��158.0g•mol-1=7.9g��
�ʴ�Ϊ��7.9��
a��������Һ����Ϊ���㡢�������ܽ⡢ת�ơ�ϴ��ת�Ƶ�����ƿ�����ݡ�ҡ�ȵȣ������õ���������������ƽ���ձ���������������ƿ����ͷ�ι� �ȣ�����Ҫ����������Ͳ����Һ�ܣ�
�ʴ�Ϊ���ۢߣ�
b������ʱ���Ӷ�����ˮ���볬���̶��ߣ��������ս��ƫС��
�ʴ�Ϊ��ƫС��
��2��MnO4+Ϊ��������������Һ��MnԪ�ػ��ϼ�+7�۱仯Ϊ+2�ۣ�H2O2 ����ԭ��������Ϊ��������ϵ����غ��ԭ���غ�õ����ӷ���ʽΪ��2MnO4-+5H2O2+6H+=2Mn2++2O2��+8H2O��
�ʴ�Ϊ��2��5��6��H+��2��5��8��H2O��
��3�����Ը��������Һ����ǿ�����ԣ��������ܣ��ζ�ʱ����������ر���Һע����ʽ�ζ��ܣ��ζ������յ�������ǣ��������һ�θ��������Һ����Һ����ɫ��Ϊdz��ɫ������ɫ������30���ڲ���ɫ��
�ʴ�Ϊ����ʽ���������һ�θ��������Һ����Һ����ɫ��Ϊdz��ɫ������ɫ������30���ڲ���ɫ��
��4��ƽ������ KMnO4����ҺV mL��
2MnO4-+5H2O2+6H+=2Mn2++2O2��+8H2O��
2 5
0.10mol•L-1 ��V��10-3L n
n=2.5V��10-4mol��
250ml��Һ�й����������ʵ���=2.5V��10-4mol��$\frac{250}{25}$=2.5V��10-3mol��
ԭ����������Һ�й����������������=$\frac{2.5V��1{0}^{-3}mol��34g/mol}{10.00mL����g/mL}$��100%=$\frac{0.0085V}{p}$��100%��
�ʴ�Ϊ��$\frac{0.0085V}{p}$��
��5������c�����⣩=$\frac{c������V������}{V�����⣩}$�жϣ����ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ����ȡ���ı���Һ������ⶨ���ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
���� ���⿼������Һ���ơ��ζ�ʵ��Ĺ��̷����ͼ���Ӧ�á�������ԭ��Ӧ�����غ�����ӷ���ʽ��д�ȣ����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 �����ܽ⡱��ͭԪ�ص���Ҫ������ʽ��Cu2+�������ӷ��ţ���
�����ܽ⡱��ͭԪ�ص���Ҫ������ʽ��Cu2+�������ӷ��ţ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ������ | Fe3+ | Fe2+ | Mg2+ | Al3+ | Cu2+ | Cr3+ |
| ��ʼ����ʱ�� pH | 1.9 | 7.0 | 9.3 | 3.7 | 4.7 | �� |
| ������ȫʱ�� pH | 3.2 | 9.0 | 11.1 | 5.2 | 6.7 | 9����9�ܽ⣩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g������H��0��ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g������H��0��ϵ�У�n��NO����ʱ��ı仯�����| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n��NO����mol�� | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 ��������ѧ������Ϊ���ۼ���
��������ѧ������Ϊ���ۼ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
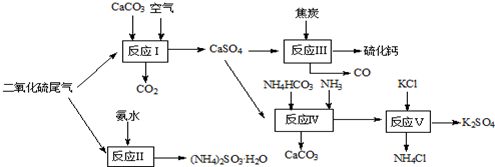
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com